 | |
| Names | |
|---|---|
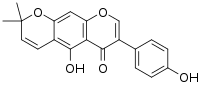
| IUPAC name
4′,5-Dihydroxy-6′′,6′′-dimethyl-6′′H-pyrano[3′′,2′′:6,7]isoflavone | |
| Systematic IUPAC name
5-Hydroxy-3-(4-hydroxyphenyl)-8,8-dimethyl-4H,8H-benzo[1,2-b:5,4-b′]dipyran-4-one | |
| Other names
5-Hydroxy-3-(4-hydroxyphenyl)-8,8-dimethylpyrano[3,2-g]chromen-4-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H16O5 | |
| Molar mass | 336.33 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Alpinumisoflavone is a pyranoisoflavone, a type of isoflavone. It can be found in the bark of Rinorea welwitschii.[1] It can also be found in the molluscicide plant Millettia thonningii and is thought to be an antischistosomal agent[2] since it has been shown to kill the snails which transmit the schistosomiasis and also the larvae of the parasite itself.[3]
References
- ↑ Pyranoisoflavones from Rinorea welwitschii. M. Stewart, B. Bartholomew, F. Currie, D. K. Abbiw, Z. Latif, S. D. Sarker and R. J. Nash, Fitoterapia, Volume 71, Issue 5, 1 September 2000, Pages 595-597, doi:10.1016/S0367-326X(00)00210-0
- ↑ The plant molluscicide Millettia thonningii (Leguminosae) as a topical antischistosomal agent. S. Perrett, P. J. Whitfield, L. Sanderson and A. Bartlett, Journal of Ethnopharmacology, Volume 47, Issue 1, 23 June 1995, Pages 49-54
- ↑ Aqueous degradation of isoflavonoids in an extract of Millettia thonningii (Leguminosae) which is larvicidal towards schistosomes. Perrett S. and Whitfield P. J., PTR. Phytotherapy, 1995, vol. 9, no6, pp. 401-404
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.