 | |
 | |
| Names | |
|---|---|
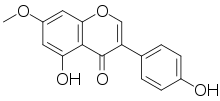
| IUPAC name
4′,5-Dihydroxy-7-methoxyisoflavone | |
| Systematic IUPAC name
5-Hydroxy-3-(4-hydroxyphenyl)-7-methoxy-4H-1-benzopyran-4-one | |
| Other names
Prunusetin 5,4'-dihydroxy-7-methoxyisoflavone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.199 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H12O5 | |
| Molar mass | 284.26 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Prunetin is an O-methylated isoflavone, a type of flavonoid. It has been isolated for the first time by Finnemore in 1910 in the bark of Prunus emarginata (the Oregon cherry).[1] Prunetin isolated from pea roots can act as an attractant for Aphanomyces euteiches zoospores.[2] It is also an allosteric inhibitor of human liver aldehyde dehydrogenase.[3]
Prunetin can lower blood pressure of spontaneously hypertensive rats and relax isolated rat aortic rings through calcium channel block mechanisms in vessel smooth muscles.[4]
Glycosides
- 8-C-glucosyl prunetin, isolated from the leaves of Dalbergia hainanensis[5]
See also
References
- ↑ Shriner, R. L.; Hull, Clarence J. (1945). "Isoflavones. III. The Structure of Prunetin and a New Synthesis of Genistein1". The Journal of Organic Chemistry. 10 (4): 288–291. doi:10.1021/jo01180a006.
- ↑ Yokosawa, Ryozo; Kuninaga, Shiro; Sekizaki, Harua (1986). "Aphanomyces euteiches zoospore attractant isolated from pea root; prunetin" (PDF). Ann. Phytopath. Soc. Japan. 52 (5): 809–816. doi:10.3186/jjphytopath.52.809. Archived from the original (PDF) on 2011-07-22.
- ↑ Sheikh, S.; Weiner, H. (1997). "Allosteric inhibition of human liver aldehyde dehydrogenase by the isoflavone prunetin". Biochemical Pharmacology. 53 (4): 471–478. doi:10.1016/s0006-2952(96)00837-4. PMID 9105397.
- ↑ Kim, B., Jo, C., Choi, H. Y., & Lee, K. (2018). "Prunetin relaxed isolated rat aortic rings by blocking calcium channels". Molecules, 23(9), 2372. doi:10.3390/molecules23092372 PMC 6225200 PMID 30227625
- ↑ Conformational Study of 8-C-glucosyl-prunetin by Dynamic NMR Spectroscopy. Pei Cheng Zhang, Ying Hong Wang, Xin Liu, Xiang Yi, Ruo Yun Chen and De Quan Yu, Chinese Chemical Letters Vol. 13, No. 7, pp 645 – 648, 2002 Archived 2011-07-07 at the Wayback Machine
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.