 | |
| Names | |
|---|---|
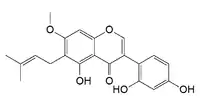
| IUPAC name
2′,4′,5-Trihydroxy-7-methoxy-6-(3-methylbut-2-en-1-yl)isoflavone | |
| Systematic IUPAC name
3-(2,4-Dihydroxyphenyl)-5-hydroxy-7-methoxy-6-(3-methylbut-2-en-1-yl)-4H-1-benzopyran-4-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H20O6 | |
| Molar mass | 368.385 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
7-O-Methylluteone is a prenylated isoflavone. It can be found in the bark of Erythrina burttii.[1]
The enzyme monoprenyl isoflavone epoxidase uses 7-O-methylluteone, NADPH, H+ and O2 to produce a dihydrofurano pyranoisoflavone derivative, NADP+ and H2O.
References
- ↑ Two prenylated flavonoids from the stem bark of Erythrina burttii. Abiy Yenesew, Beatrice Irungu, Solomon Derese, Jacob O. Midiwo, Matthias Heydenreich and Martin G. Peter, Phytochemistry, Volume 63, Issue 4, June 2003, Pages 445–448, doi:10.1016/S0031-9422(03)00209-7
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.