 | |
| Clinical data | |
|---|---|
| Other names |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
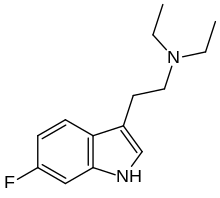
| Formula | C14H19FN2 |
| Molar mass | 234.318 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
6-Fluoro-DET (6F-DET, 6-fluoro-N,N-diethyltryptamine) is a substituted tryptamine derivative related to drugs such as DET and 5-fluoro-DET. It acts as a partial agonist at the 5-HT2A receptor, but while it produces similar physiological effects to psychedelic drugs, it does not appear to produce psychedelic effects itself even at high doses. For this reason it saw some use as an active placebo in early clinical trials of psychedelic drugs but was regarded as having little use otherwise,[1] though more recent research into compounds such as AL-34662, TBG and AAZ-A-154 has shown that these kind of non-psychedelic 5-HT2A agonists can have various useful applications.[2][3][4][5][6][7]
See also
References
- ↑ Faillace LA, Vourlekis A, Szara S (October 1967). "Clinical evaluation of some hallucinogenic tryptamine derivatives". The Journal of Nervous and Mental Disease. 145 (4): 306–313. doi:10.1097/00005053-196710000-00005. PMID 6076017. S2CID 19328310.
- ↑ Martin WR, Sloan JW (1977). "Pharmacology and Classification of LSD-like Hallucinogens". In Martin WR (ed.). Drug Addiction II. Handbuch der experimentellen Pharmakologie. Handbook of Experimental Pharmacology. Vol. 45. Berlin, Heidelberg: Springer. pp. 305–368. doi:10.1007/978-3-642-66709-1_3. ISBN 978-3-642-66711-4.
- ↑ Blair JB, Kurrasch-Orbaugh D, Marona-Lewicka D, Cumbay MG, Watts VJ, Barker EL, Nichols DE (November 2000). "Effect of ring fluorination on the pharmacology of hallucinogenic tryptamines". Journal of Medicinal Chemistry. 43 (24): 4701–4710. doi:10.1021/jm000339w. PMID 11101361.
- ↑ Rabin RA, Regina M, Doat M, Winter JC (May 2002). "5-HT2A receptor-stimulated phosphoinositide hydrolysis in the stimulus effects of hallucinogens". Pharmacology, Biochemistry, and Behavior. 72 (1–2): 29–37. doi:10.1016/s0091-3057(01)00720-1. PMID 11900766. S2CID 6480715.
- ↑ Nichols DE (2018). "Chemistry and Structure-Activity Relationships of Psychedelics". Current Topics in Behavioral Neurosciences. 36: 1–43. doi:10.1007/7854_2017_475. ISBN 978-3-662-55878-2. PMID 28401524.
- ↑ Cameron LP, Tombari RJ, Lu J, Pell AJ, Hurley ZQ, Ehinger Y, et al. (January 2021). "A non-hallucinogenic psychedelic analogue with therapeutic potential". Nature. 589 (7842): 474–479. Bibcode:2021Natur.589..474C. doi:10.1038/s41586-020-3008-z. PMC 7874389. PMID 33299186.
- ↑ Dong C, Ly C, Dunlap LE, Vargas MV, Sun J, Hwang IW, et al. (May 2021). "Psychedelic-inspired drug discovery using an engineered biosensor". Cell. 184 (10): 2779–2792.e18. doi:10.1016/j.cell.2021.03.043. PMC 8122087. PMID 33915107.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.