 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.164.424 |
| Chemical and physical data | |
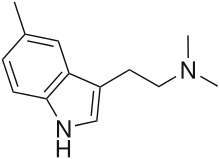
| Formula | C13H18N2 |
| Molar mass | 202.301 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
5,N,N-trimethyltryptamine (5,N,N-TMT; 5-TMT) is a tryptamine derivative that is a psychedelic drug. It was first made in 1958 by Edwin H. P. Young.[1] In animal experiments it was found to be in between DMT and 5-MeO-DMT in potency[2][3] which would suggest an active dosage for humans in the 20–60 mg range. Human psychoactivity for this compound has been claimed in reports on websites such as Erowid but has not been independently confirmed.
Legal Status
United States
5,N,N-TMT is not scheduled at the federal level in the United States,[4][5] but it could be considered an analog of 5-MeO-DMT, in which case, sales or possession intended for human consumption could be prosecuted under the Federal Analog Act.
See also
References
- ↑ Young EH (1958). "704. The synthesis of 5-hydroxytryptamine (serotonin) and related tryptamines". Journal of the Chemical Society (Resumed): 3493–6. doi:10.1039/JR9580003493.
- ↑ Glennon RA, Gessner PK (April 1979). "Serotonin receptor binding affinities of tryptamine analogues". Journal of Medicinal Chemistry. 22 (4): 428–32. doi:10.1021/jm00190a014. PMID 430481.
- ↑ Glennon RA, Young R, Rosecrans JA, Kallman MJ (1980). "Hallucinogenic agents as discriminative stimuli: a correlation with serotonin receptor affinities". Psychopharmacology. 68 (2): 155–8. doi:10.1007/BF00432133. PMID 6776558. S2CID 1674481.
- ↑ "§ 1308.11 Schedule I." Archived from the original on 2009-08-27. Retrieved 2014-12-17.
- ↑ "§ 1308.11 Schedule I." e-CFR. 2022-02-17. Archived from the original on 2022-02-10.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.