 | |
| Names | |
|---|---|
| IUPAC name
Gallium rhenate(VII) | |
| Other names
Gallium metaperrhenate | |
| Identifiers | |
| |
3D model (JSmol) |
|
| |
| |
| Properties | |
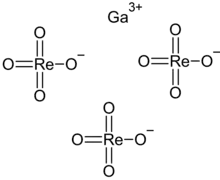
| Ga(ReO4)3 | |
| Molar mass | 820.344 |
| Appearance | White deliquescent crystals (anhydrous)[1] |
| Soluble | |
| Related compounds | |
Other anions |
Gallium nitrate Gallium perchlorate |
Other cations |
Potassium perrhenate |
Related compounds |
Rhenium(VII) oxide Perrhenic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Gallium perrhenate is an inorganic compound with the chemical formula of Ga(ReO4)3. It exists in the anhydrous and hydrate forms.
Preparation
Gallium can be anodically dissolved in perrhenic acid at 50-55 °C, and dried with sulfuric acid to obtain the hydrate crystal [Ga(H2O)6(ReO4)3]·2H2O.[2] The anhydrous form can be obtained by reacting gallium(III) oxide and rhenium(VII) oxide at 450 °C.[1]
Chemical properties
Gallium perrhenate decomposes above 300 °C to form rhenium(VII) oxide and gallium(III) oxide:[3]
- 2 Ga(ReO4)3 → 3 Re2O7 + Ga2O3
References
- 1 2 Baud, Gilbert; Capestan, Michel (1968). "Gallium perrhenate and its hydrates". Comptes Rendus de l'Académie des Sciences, Série C (in French). 266 (6): 382–384. ISSN 0567-6541.
- ↑ Vol'fkovich, A. Yu.; Khrustalev, V. N.; Shamrai, N. B.; Varfolomeev, M. B. (1997). "Electrochemical synthesis of aluminum, gallium, and indium perrhenates". Zhurnal Neorganicheskoi Khimii (in Russian). 42 (12): 1960–1962. ISSN 0044-457X.
- ↑ 谢高阳 等主编 (ed.). "3.13.3 含氧酸及其盐类". 第九卷 锰分族 铁系 铂系 [Volume IX Manganese Group Iron Series Platinum Series]. 无机化学丛书. 科学出版社. p. 116.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.