 | |
 | |
| Names | |
|---|---|
| IUPAC name
trimethylgallane, trimethanidogallium | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.014.452 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Ga(CH3)3 | |
| Molar mass | 114.827 g/mol |
| Appearance | colourless liquid |
| Melting point | −15 °C (5 °F; 258 K) |
| Boiling point | 55.7 °C (132.3 °F; 328.8 K) |
| Reacts with water | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Pyrophoric (can ignite spontaneously in air), reacts with water to release methane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
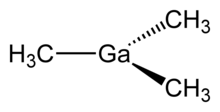
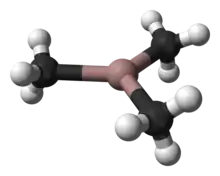
Trimethylgallium, often abbreviated to TMG or TMGa, is the organogallium compound with the formula Ga(CH3)3. It is a colorless, pyrophoric liquid.[1] Unlike trimethylaluminium, TMG adopts a monomeric structure.[2] When examined in detail, the monomeric units are clearly linked by multiple weak Ga---C interactions, reminiscent of the situation for trimethylindium.[3]
Preparation
Two forms of TMG are typically investigated: Lewis base adducts or TMG itself. All are prepared by reactions of gallium trichloride with various methylating agents. When the methylation is conducted with methylmagnesium iodide in diethyl ether, the product is the poorly volatile diethyl ether adduct. The ether ligand is not readily lost, although it may be displaced with liquid ammonia.[4] When the alkylation is conducted with methyl lithium in the presence of a tertiary phosphine the air-stable phosphine adduct is obtained:
- GaCl3 + 3 MeLi + PR3 → R3P−GaMe3 + 3 LiCl
Heating the solid phosphine adduct under vacuum liberates the base-free TMG:[1]
- R3P−GaMe3 → R3P + GaMe3
Other non-volatile bases have been described.[5] Other methylating agents for the synthesis of TMG include dimethylzinc and trimethylaluminium.
Applications
TMG is the preferred metalorganic source of gallium for metalorganic vapour phase epitaxy (MOVPE) of gallium-containing compound semiconductors, such as GaAs, GaN, GaP, GaSb, InGaAs, InGaN, AlGaInP, InGaP, AlInGaNP and Ga2O3.[6] These material are used in the production of LED lighting and semiconductors as a metalorganic chemical vapor deposition precursor.
References
- 1 2 Bradley, D. C.; Chudzynska, H. C.; Harding, I. S. (1997). "Trimethylindium and Trimethylgallium". Inorganic Syntheses. 31: 67–74. doi:10.1002/9780470132623.ch8. ISBN 9780470132623.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Mitzel, Norbert W.; Lustig, Christian; Berger, Raphael J. F.; Runeberg, Nino (2002). "Luminescence Phenomena and Solid-State Structures of Trimethyl- and Triethylgallium". Angewandte Chemie International Edition. 41 (14): 2519–2522. doi:10.1002/1521-3773(20020715)41:14<2519::AID-ANIE2519>3.0.CO;2-2.
- ↑ Kraus, C. A.; Toonder, F. E. (1933). "Trimethyl Gallium, Trimethyl Gallium Etherate and Trimethyl Gallium Ammine". PNAS. 19 (3): 292–8. Bibcode:1933PNAS...19..292K. doi:10.1073/pnas.19.3.292. PMC 1085965. PMID 16577510.
- ↑ Foster, Douglas F.; Cole-Hamilton, David J. (1997). "Electronic Grade Alkyls of Group 12 and 13 Elements". Inorganic Syntheses. 31: 29-66. doi:10.1002/9780470132623.ch7.
- ↑ Shenai-Khatkhate, D. V.; Goyette, R. J.; Dicarlo, R. L. Jr; Dripps, G. (2004). "Environment, health and safety issues for sources used in MOVPE growth of compound semiconductors". Journal of Crystal Growth. 272 (1–4): 816–21. Bibcode:2004JCrGr.272..816S. doi:10.1016/j.jcrysgro.2004.09.007.