| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
(Trichloromethyl)benzene | |||
| Other names
Toluene trichloride Phenyl chloroform α,α,α-Trichlorotoluene PhCCl3 | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.395 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2226 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C7H5Cl3 | |||
| Molar mass | 195.48 | ||
| Appearance | Clear liquid | ||
| Odor | unpleasant | ||
| Density | 1.3756 g/mL | ||
| Melting point | −5.0 °C (23.0 °F; 268.1 K) | ||
| Boiling point | 220.8 °C (429.4 °F; 493.9 K) | ||
| 0.05g/L | |||
| Solubility | organic solvents | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
potential occupational carcinogen | ||
| GHS labelling: | |||
   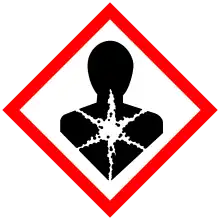 | |||
| Danger | |||
| H302, H305, H315, H318, H331, H335, H350 | |||
| P201, P202, P261, P264, P270, P271, P280, P281, P301+P312, P302+P352, P304+P340, P305+P351+P338, P308+P313, P310, P311, P312, P321, P330, P332+P313, P362, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 97.22 °C (207.00 °F; 370.37 K) | ||
| 420 °C (788 °F; 693 K) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
120 mg/kg (in rats) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Benzotrichloride (BTC), also known as α,α,α-trichlorotoluene, phenyl chloroform or (trichloromethyl)benzene, is an organic compound with the formula C6H5CCl3. Benzotrichloride is an unstable, colorless or somewhat yellowish, viscous, chlorinated hydrocarbon with a penetrating odor. Benzotrichloride is used extensively as a chemical intermediate for products of various classes, i.e. dyes and antimicrobial agents.[1]
Structure and reactivity
Benzotrichloride is a poorly water-soluble, clear to yellowish liquid with a penetrating odor. It hydrolyzes rapidly to benzoic acid and hydrochloric acid with a half life of about 2.4 minutes, thus making the compound unstable in the presence of water.[2] In other chemical reactions, benzotrichloride reacts at the chlorinated α-carbon, for example in substitution reactions. It is used as an intermediate in the synthesis of benzoyl chloride, benzotrifluoride and 2,4-dihydroxybenzophenone which in turn are also intermediates in other reactions:[2]
- C6H5CCl3 + H2O → C6H5C(O)Cl + 2 HCl[5]
- C6H5CCl3 + 3 KF → C6H5CF3 + 3 KCl[6]
These compounds are further used to synthesize chemicals needed in the pharmaceutical industry (benzoyl peroxide), the synthesis of pesticides, dyes and UV-absorbing compounds which are often used in paint and plastics to prevent degradation by sunlight.[2]
Production
Production capacity of benzotrichloride was estimated at 80,000 tonnes for the year 2000. It is produced by the free radical chlorination of toluene, catalysed by light or radical initiators such as dibenzoyl peroxide. Mono- and di-chlorinated intermediates are observed as benzyl chloride and benzal chloride:[2]
Regulation
Benzotrichloride is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and hence its use is subject to a list of reporting requirements by companies or institutions which synthesize, store or use it in large quantities. In 2018, EU member states have approved a European Commission proposal to restrict the use of carcinogenic, mutagenic and reprotoxic (CMR) substances in clothing, textiles and footwear. In 2015, the Commission published a preliminary list of 286 CMRs it proposed to restrict. Benzotrichloride was listed in this document as a toxic and carcinogenic compound.[7]
According to the harmonised classification and labelling (CLP00) approved by the European Union (EU), this substance is toxic if inhaled, causes serious eye damage, may cause cancer, causes skin irritation, is harmful if swallowed, and may cause respiratory irritation.[8]
Metabolism
In a rat experiment with radiolabeled [14C]-benzotrichloride a single 40 mg/kg body weight dose was administered. The absorption half-life of BTC from the gastrointestinal tract was determined to be 3 hours. The concentration in the blood peaked at 4 h reaching 6.5 ppm, this decreased to 2.6 ppm after 24 h. The elimination of half-life in blood was 22 h. Elimination took place for 90% through urine and 10% through faeces. After 72 hours 1.5% of the dose was still present in tissue. The highest concentration levels were present in liver, kidney and fat.[2]
BTC is rapidly metabolised via hydrolysis to benzoic acid and hydrochloric acid. This benzoic acid is first metabolized into benzoyl-CoA, which is metabolized into hippuric acid by replacing CoA with glycine. This hippuric acid is then excreted.[9] 90% of the BTC was recovered from the rat urine as hippuric acid while small amounts of benzoic acid (0.7%) and phenyl acetic acid (0.8%) were also present. Four unidentified metabolites (5.5%) were also present in urine.[2]
Toxicity
Signs of toxicity
Several symptoms are related to the tested exposure to benzotrichloride (BTC) in rats: irritation of the eyes, the skin and the respiratory tract. Under occlusive conditions, rabbit skin which was exposed to BTC showed irritation. Next, severe eye irritation was reported in rabbits, after administering 0.1 mL of BTC. This eye irritation lasted up to 7 days. Finally, several rat studies into the acute toxic effects indicate that the respiratory system will be irritated after inhalation or oral uptake of BTC.[2]
The effects of repeated inhalation, estimated with experiments on rats, include the following. BTC can lead to bronchitis and bronchopneumonia, depressed weight gain and gasping. Microscopically, inflammation and squamous metaplasia of the cells lining the nasal, tracheal, bronchial and bronchiolar epithelium can occur in rats. Histopathologically, an increased incidence of portal inflammatory cells infiltrate the liver and also bile duct proliferation is likely to occur.[10]
The toxicity of BTC in mammals was assessed by Chu I. et al. (1984) by tracking several characteristics in rats for 28 days after oral intake of BTC. Growth rate and food consumption were not found to be affected by treatment. No deaths occurred during these trials.[11]
Animal toxicity
Acute toxicity
Inhalation
Inhaling 1147 mg/m3 of benzotrichloride (BTC) for 3 hours resulted in 1 out of 6 male rats dying (after 3 days). On the other hand, inhaling 995 mg/m3 of BTC for 3 hours resulted in 4 out of 6 female rats dying within 13 days. However, reducing the duration of exposure to only one hour with a concentration just above 800 mg/m3 resulted in no male or female rats dying. The treated rats had irritated oral and ocular mucous membranes, while behaviour and breathing were altered for up to 13 days.[2]
Dermal
Out of 10 female rats 1 died after being exposed to 5000 mg/kg body weight of BTC. This indicates that the median lethal dosage, the LD50 value, is higher than 5000 mg/kg body weight. The rats showed sedation starting from day 1 until day 8-10.[2]
Oral
Studying 15 male and 15 female rats per dose group, after being given an oral dosage, an LD50 of 2188 mg/kg bodyweight and an LD50 of 1590 mg/kg bodyweight were found for males and females, respectively. Laboured breathing, polyuria, sanguineous urine, and reduced activity were observed, lasting from 15 minutes after ingestion until 7–9 days. The deceased rats had empty intestinal tracts and white-stippled stomachs.[12][2] Moreover, pure compound administration resulted in a male LD50 of 1249 mg/kg body weight. Symptoms in these male rats included: bloody eyes, ataxia, cramps, diuresis, weight loss.[2] Another study found an LD50 of 770 mg/kg (male) and 702 mg/kg (female) after administering these rats with benzoyl chloride in corn oil. Besides the same symptoms described earlier, upon necropsy lung congestion, thymus with red foci and yellow stained urogenital region and fluid filled intestines were found.[13]
Repeated dose toxicity
Inhalation
The toxic effects of repeated benzotrichloride exposure have been assessed for inhalation, dermal exposure and oral ingestion. After repeated exposure to a concentration of 12.8 mg/m3 twice weekly for 30 minutes, over 12 months in mice, severe bronchitis and bronchial pneumonia were observed.[14] After exposure of 5.1 mg/m3 for 6 hours a day, 5 days a week for 4 weeks, no adverse effects were observed in rats.[13] Note that the exposure times resemble a 5-day work week (although with only 30 hours).
Dermal
After dermal administration in rabbits between 50 and 200 mg/kg bodyweight per day (5 days a week, 3 weeks) skin irritation up to necrosis was observed, suggesting that it is a dermal irritant.[2]
Oral
After feeding rats 0.048–53 mg/kg body weight for 28 days, only mild microscopical liver kidney and thyroid structure change was observed, even at the lowest dose.[11] The data presented in this study suggest that BTC possess a low order of oral toxicity in the rat.[11]
Mutagenicity and carcinogenicity
Inhalation
Genotoxic potential was shown in bacteria and mammalian cell system, micronucleus formation occurred in bone marrow cells. Actual cancer incidence increase was clearly observed too: The same study finding bronchitis after long term respiratory use, found that 81% of mice had lung adenomas(8% in control), 27% skin adenomas (0% in control), 11% malignant lymphomas (0% in control).[14] Similar studies also showed significant carcinogenicity.[2] This shows that even though long-term inhalation damages the lungs, it also increases cancer risk, which is a risk needing stricter regulation.
Dermal
Dermal and oral studies all showed significant lung cancer increase, together with either significant skin cancer and stomach cancer increase respectively.[15] Research, after suspicion of carcinogenicity in benzoyl-chloride producing factories, on ICR mice also showed significant incidence of tumors: skin-cancer(68%) and pulmonary tumors(58%) after applying 2.3 microliter/animal twice weekly for 50 weeks.[16]
Oral
In humans only a few cases of lung cancer are linked to either benzoyl chloride or benzotrichloride, although smoking also might have played a role.[17] Both NCI thesaurus and NPT carcinogen reports classify BTC as “reasonably anticipated to be a human carcinogen”, based on limited evidence of carcinogenicity from studies in humans and sufficient evidence of carcinogenicity from studies in experimental animals.[18][19]
Aquatic effects
Daphnia magna (a planktonic crustacean) were tested, 24 h-EC50 of 50 mg/L was found. The toxic effects were attributed to HCl formation, as benzotrichloride dissociates rapidly into the far less toxic benzoic acid and HCl on water exposure. Compensating the pH decrease in water from the HCl negated toxic effects, suggesting that water acidification is the reason for the low aquatic life toxicity.[20] Benzoic acid has an EC50 of >100 mg/L towards aquatic life, is readily biodegradable and does not accumulate, so is not regarded as toxic towards aquatic life.
Fertility
No extensive studies were done on fertility effects. As the carcinogenic potential on its own already warrants extensive restrictions, no additional restrictions are needed for effect on fertility, although the genotoxic properties suggest that fertility might be affected.[2]
Mechanism of toxicity
Part of the toxicity of benzotrichloride can be explained by its hydrolysis to benzoic acid, whose further metabolism can cause toxic effects. Benzoyl-CoA formation can deplete acetyl-CoA levels, hampering processes requiring acetyl-CoA, like gluconeogenesis via pyruvate carboxylase.[21] Hepatic ATP levels are also lowered by 70-80%, at doses of 720–1440 mg/kg benzoic acid via intraperitoneal injection, by reducing acetyl-CoA availability for ATP production, which can have a wide range of consequences for affected cells.[21] Ammonia toxicity can be amplified by benzoic acid, as it inhibits ureagenesis, reducing ammonia detoxification. Furthermore, benzoic acid can displace bilirubin from the albumins, posing a risk of bilirubin toxicity, as it diffuses into tissues.[21]
Benzoic acid was shown to be genotoxic in vitro.[22] Benzoic acid therefore might have a role in benzotrichloride carcinogenicity, but benzotrichloride has more carcinogenic potential than benzoic acid, suggesting that an intermediate in hydrolysis is responsible for at least part of the carcinogenicity.[1] Research showed that the mutagenicity was not caused by reactive oxygen species (ROS) increase, but it did not elucidate what metabolite was the main carcinogen.[23]
References
- 1 2 Yasuo, Kimie; Fujimoto, Sachiko; Katoh, Masanobu; Kikuchi, Yoshiaki; Kada, Tsuneo (1978-11-01). "Mutagenicity of benzotrichloride and related compounds". Mutation Research/Genetic Toxicology. 58 (2): 143–150. doi:10.1016/0165-1218(78)90003-4. ISSN 0165-1218. PMID 106269.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 "α,α,α-Trichlorotoluene (Trichloromethylbenzene)". OECD Sids. 2004-01-23.
- ↑ Ponder, Fernando J (12 January 1968). "Synthesis of 2-hydroxy-4-alkoxybenzophenones" (PDF). United States Patent Office.
- ↑ PubChem. "2,4-Dihydroxybenzophenone". pubchem.ncbi.nlm.nih.gov. Retrieved 2020-03-20.
- ↑ Rossberg, Manfred; Lendle, Wilhelm; Pfleiderer, Gerhard; Tögel, Adolf; Dreher, Eberhard-Ludwig; Langer, Ernst; Rassaerts, Heinz; Kleinschmidt, Peter; Strack, Heinz; Cook, Richard; Beck, Uwe; Lipper, Karl-August; Torkelson, Theodore R.; Löser, Eckhard; Beutel, Klaus K.; Mann, Trevor (2006). "Chlorinated Hydrocarbons". Ullmann's Encyclopedia of Industrial Chemistry: 139. doi:10.1002/14356007.a06_233.pub2. ISBN 3527306730.
- ↑ Bonath, B.; Förtsch, B.; Saemann, R. (1966). "Kinetische Untersuchung einer Seitenkettenchlorierung unter Verwendung eines Analogcomputers". Chemie Ingenieur Technik. 38 (7): 739–742. doi:10.1002/cite.330380711.
- ↑ "EU member states back CMR restrictions in clothing, textiles". Chemical Watch. Retrieved 2020-03-20.
- ↑ "α,α,α-trichlorotoluene - Substance Information - ECHA". echa.europa.eu. Retrieved 2020-03-20.
- ↑ Irwin, Cindy; Reenen, Mari van; Mason, Shayne; Mienie, Lodewyk J.; Westerhuis, Johan A.; Reinecke, Carolus J. (2016-12-01). "Contribution towards a Metabolite Profile of the Detoxification of Benzoic Acid through Glycine Conjugation: An Intervention Study". PLOS ONE. 11 (12): e0167309. Bibcode:2016PLoSO..1167309I. doi:10.1371/journal.pone.0167309. ISSN 1932-6203. PMC 5132330. PMID 27907139.
- ↑ Warheit, D. B.; Carakostas, M. C.; Kelly, D. P.; Hartsky, M. A. (April 1991). "Four-week inhalation toxicity study with Ludox colloidal silica in rats: pulmonary cellular responses". Fundamental and Applied Toxicology. 16 (3): 590–601. doi:10.1016/0272-0590(91)90098-o. ISSN 0272-0590. PMID 1649779.
- 1 2 3 Chu, I.; Shen, S. Y.; Villeneuve, D. C.; Secours, V. E.; Valli, V. E. (March 1984). "Toxicity of trichlorotoluene isomers: a 28-day feeding study in the rat". Journal of Environmental Science and Health. Part. B, Pesticides, Food Contaminants, and Agricultural Wastes. 19 (2): 183–191. doi:10.1080/03601238409372424. ISSN 0360-1234. PMID 6736562.
- ↑ "α,α,α-trichlorotoluene - Registration Dossier - ECHA". echa.europa.eu (in Dutch). Retrieved 2020-03-20.
- 1 2 Takahashi, N.; Kadota, T.; Kawano, S.; Ishikawa, K.; Kuroyanagi, K.; Hamajima, Y.; Ohta, K.; Ohta, S.; Kai, S.; Kohmura, H. (April 1986). "[Toxicity studies of VP 16-213 (I)--Acute toxicity in mice, rats and rabbits]". The Journal of Toxicological Sciences. 11 Suppl 1: 1–16. doi:10.2131/jts.11.supplementi_1. ISSN 0388-1350. PMID 3761389.
- 1 2 Yoshimura, H.; Takemoto, K.; Fukuda, K.; Matsushita, H. (September 1986). "[Carcinogenicity in mice by inhalation of benzotrichloride and benzoyl chloride]". Sangyo Igaku. Japanese Journal of Industrial Health. 28 (5): 352–359. doi:10.1539/joh1959.28.352. ISSN 0047-1879. PMID 3820773.
- ↑ Fukuda, K.; Matsushita, H.; Sakabe, H.; Takemoto, K. (October 1981). "Carcinogenicity of benzyl chloride, benzal chloride, benzotrichloride and benzoyl chloride in mice by skin application". Gan. 72 (5): 655–664. ISSN 0016-450X. PMID 7327367.
- ↑ "Carcinogenicity of benzyl chloride, benzal chloride, benzotrichloride and benzoyl chloride in mice by skin application. | Sigma-Aldrich". www.sigmaaldrich.com. Retrieved 2020-03-20.
- ↑ Sakabe, Hiroyuki; Matsushita, Hidetsuru; Koshi, Shigezi (1976). "Cancer Among Benzoyl Chloride Manufacturing Workers". Annals of the New York Academy of Sciences. 271 (1): 67–70. Bibcode:1976NYASA.271...67S. doi:10.1111/j.1749-6632.1976.tb23094.x. ISSN 1749-6632. PMID 1069541. S2CID 26430038.
- ↑ "NCI Thesaurus". ncit.nci.nih.gov. Retrieved 2020-03-20.
- ↑ Administrator. "Acrobat Accessibility Report" (PDF). ntp.niehs.nih.gov. Retrieved 2020-03-20.
- ↑ Bringmann G and Kuehn R (1977). Befunde der Schadwirkung wassergefaehrdender Stoffe gegen Daphnia magna. Z. Wasser- Abwasser-Forsch. pp. 10 (5), 161–166.
- 1 2 3 Tremblay, George C.; Qureshi, Ijaz A. (1993-01-01). "The biochemistry and toxicology of benzoic acid metabolism and its relationship to the elimination of waste nitrogen". Pharmacology & Therapeutics. 60 (1): 63–90. doi:10.1016/0163-7258(93)90022-6. ISSN 0163-7258. PMID 8127924.
- ↑ Yılmaz, Serkan; Ünal, Fatma; Yüzbaşıoğlu, Deniz (2009-07-30). "The in vitro genotoxicity of benzoic acid in human peripheral blood lymphocytes". Cytotechnology. 60 (1): 55. doi:10.1007/s10616-009-9214-z. ISSN 1573-0778. PMC 2780543. PMID 19642007.
- ↑ Yamamoto, Kimiyo N.; Hirota, Kouji; Kono, Koichi; Takeda, Shunichi; Sakamuru, Srilatha; Xia, Menghang; Huang, Ruili; Austin, Christopher P.; Witt, Kristine L.; Tice, Raymond R. (2011). "Characterization of environmental chemicals with potential for DNA damage using isogenic DNA repair-deficient chicken DT40 cell lines". Environmental and Molecular Mutagenesis. 52 (7): 547–561. doi:10.1002/em.20656. ISSN 1098-2280. PMC 3278799. PMID 21538559.