| Maleylacetoacetate Isomerase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 This represents the surface structure of the enzyme. | |||||||||
| Identifiers | |||||||||
| EC no. | 5.2.1.2 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
In enzymology, maleylacetoacetate isomerase (EC 5.2.1.2) is an enzyme that catalyzes the chemical reaction
- 4-maleylacetoacetate 4-fumarylacetoacetate
This enzyme belongs to the family of isomerases, specifically cis-trans isomerases. The systematic name of this enzyme class is 4-maleylacetoacetate cis-trans-isomerase.
4-Maleylacetoacetate isomerase is an enzyme involved in the degradation of L-phenylalanine. It is encoded by the gene glutathione S-transferase zeta 1, or GSTZ1. This enzyme catalyzes the conversion of 4-maleylacetoacetate to 4-fumarylacetoacetate. 4-Maleylacetoacetate isomerase belongs to the zeta class of the glutathione S-transferase (GST) superfamily.[1]

Mechanism
In the phenylalanine degradation pathway, 4-maleylacetoacetate isomerase catalyzes a cis-trans isomerization of 4-maleylacetoacetate to fumarylacetoacetate. 4-maleylacetoacetate isomerase requires the cofactor glutathione to function.[1] Ser 15, Cys 16, Gln 111, and the helix dipole of alpha 1 of the enzyme stabilize the thiolate form of glutathione which activates it to attack the alpha carbon of 4-maleylacetoacetate, thus breaking the double bond and allowing rotation around the single bond.[1]
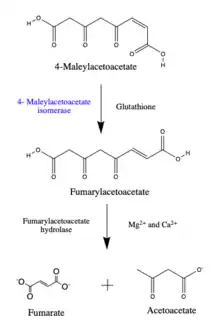
4-maleylacetoacetate is converted to 4-fumarylacetoacetate, this compound can be broken down into fumarate and acetoacetate by the enzyme fumarylacetoacetate hydrolase.[2]
The conversion of 4-maleylacetoacetate to fumarylacetoacetate is a step in the catabolism of phenylalanine and tyrosine, amino acids acquired through dietary protein consumption. When 4-maleylacetoacetate isomerase is unable to function properly, the 4-maleylacetoacetate may be converted instead to succinylacetoacetate and further broken down into succinate and acetoacetate by fumarylacetoacetate hydrolase.[3]

Structure

4-maleylacetoacetate is a homodimer. It is classified as an isomerase transferase. It has a total residue count of 216 and a total atom count of 1700. This enzyme's theoretical weight is 24.11 KDa.[1] 4-maleylacetoacetate isomerase has 3 isoforms[4] The most common isoform has two domains, the N-terminal domain (4-87) the C terminal domain (92-212) and the glutathione binding site (14-19, 71-72 and 115-117). The N-terminal domain has a four stranded beta sheet which is sandwiched by alpha helices on both sides to form a three layer sandwich tertiary structure. The C terminal domain is composed mostly of alpha helices and has an up down structure of tightly bundled alpha helices.[1]
Glutathione binds in positions 14-19, 71-72, and 115-117. It also binds the sulfate ion and dithiothreitol.[3]
Clinical significance
Maleylacetoacetate isomerase deficiency is a disease caused by a mutation in the gene GSTZ1.
This is an autosomal recessive inborn error of metabolism. It is caused by a mutation in the gene that codes for the synthesis of 4-maleylacetoacetate isomerase, GSTZ1.
Mutations in 4-maleylacetoacetate isomerase resulted in accumulation of fumarylacetoacetate and succinylacetone in the urine, but individuals were otherwise healthy. It is likely that there exists an alternate nonenzymatic bypass that allows the catabolism of 4-maleylacetoacetate in the absence of 4-maleylacetoacetate isomerase. Because of this mechanism, a mutation in the gene encoding 4-Maleylacetoacetate isomerase is not considered dangerous.[5]
GSTZ1 is highly expressed in the liver, however mutations in this gene do not impair liver function or coagulation.[6]
Gene expression
The gene from which this enzyme is synthesized is mostly expressed in the liver, with some expression in the kidneys, skeletal muscle, and brain. It is also expressed in melanocytes, synovium, placenta, breasts, fetal liver and heart.[6]

Related enzymes
Other enzymes involved in the catabolism of phenylalanine include phenylalanine hydroxylase, aminotransferase, p-hydroxyphenylpyruvate dioxygenase, homogentisate oxidase, and fumarylacetoacetate hydrolase. Mutations in some of these enzymes can lead to more severe diseases such as, phenylketonuria, alkaptonuria, and tyrosinemia.
The gene GSTZ1 is located on chromosome 14q24.3.[7]
References
- 1 2 3 4 5 PDB: 1FW1; Polekhina G, Board PG, Blackburn AC, Parker MW (February 2001). "Crystal structure of maleylacetoacetate isomerase/glutathione transferase zeta reveals the molecular basis for its remarkable catalytic promiscuity". Biochemistry. 40 (6): 1567–76. doi:10.1021/bi002249z. PMID 11327815.
- ↑ Arias-Barrau E, Olivera ER, Luengo JM, Fernández C, Galán B, García JL, Díaz E, Miñambres B (August 2004). "The homogentisate pathway: a central catabolic pathway involved in the degradation of L-phenylalanine, L-tyrosine, and 3-hydroxyphenylacetate in Pseudomonas putida". Journal of Bacteriology. 186 (15): 5062–77. doi:10.1128/JB.186.15.5062-5077.2004. PMC 451635. PMID 15262943.
- 1 2 Fernández-Cañón JM, Baetscher MW, Finegold M, Burlingame T, Gibson KM, Grompe M (July 2002). "Maleylacetoacetate isomerase (MAAI/GSTZ)-deficient mice reveal a glutathione-dependent nonenzymatic bypass in tyrosine catabolism". Molecular and Cellular Biology. 22 (13): 4943–51. doi:10.1128/MCB.22.13.4943-4951.2002. PMC 133921. PMID 12052898.
- ↑ Universal protein resource accession number GSTZ1 for "Maleylacetoacetate isomerase" at UniProt.
- ↑ Lim CE, Matthaei KI, Blackburn AC, Davis RP, Dahlstrom JE, Koina ME, Anders MW, Board PG (August 2004). "Mice deficient in glutathione transferase zeta/maleylacetoacetate isomerase exhibit a range of pathological changes and elevated expression of alpha, mu, and pi class glutathione transferases". The American Journal of Pathology. 165 (2): 679–93. doi:10.1016/S0002-9440(10)63332-9. PMC 1618558. PMID 15277241.
- 1 2 "GSTZ1 glutathione S-transferase zeta 1 [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2018-12-06.
- ↑ "Symbol report for GSTZ1". www.genenames.org.
Further reading
- "3,5-Dioxooctanedioic acid". PubChem Compound Database. National Center for Biotechnology Information. U.S. National Library of Medicine.
- Blackburn AC, Woollatt E, Sutherland GR, Board PG (1998). "Characterization and chromosome location of the gene GSTZ1 encoding the human Zeta class glutathione transferase and maleylacetoacetate isomerase". Cytogenetics and Cell Genetics. 83 (1–2): 109–14. doi:10.1159/000015145. PMID 9925947. S2CID 22835884.
- Universal protein resource accession number O43708 for "Maleylacetoacetate isomerase" at UniProt.
- "Glutathione". PubChem Compound Database. National Center for Biotechnology Information. U.S. National Library of Medicine.