 | |
| Clinical data | |
|---|---|
| Other names | α-Dihydroequilin; 7-Dehydro-17α-estradiol; Estra-1,3,5(10),7-tetraen-3,17α-diol |
| Routes of administration | By mouth |
| Drug class | Estrogen |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.010.440 |
| Chemical and physical data | |
| Formula | C18H22O2 |
| Molar mass | 270.372 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
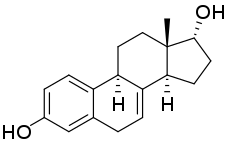
17α-Dihydroequilin, or α-dihydroequilin, also known as 7-dehydro-17α-estradiol, as well as estra-1,3,5(10),7-tetraene-3,17α-diol, is a naturally occurring steroidal estrogen found in horses which is closely related to equilin, equilenin, and 17α-estradiol.[1][2] The compound, as the 3-sulfate ester sodium salt, is present in conjugated estrogens (Premarin), a pharmaceutical extract of the urine of pregnant mares, and is the third highest quantity constituent in the formulation (13.8%).[1] The compound has been studied clinically.[3]
See also
References
- 1 2 Fritz MA, Speroff L (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 751–. ISBN 978-1-4511-4847-3.
- ↑ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 378–. ISBN 978-92-832-1291-1.
- ↑ Wilcox JG, Stanczyk FZ, Morris RS, Gentzschein E, Lobo RA (November 1996). "Biologic effects of 17 alpha-dihydroequilin sulfate". Fertility and Sterility. 66 (5): 748–52. doi:10.1016/S0015-0282(16)58629-4. PMID 8893678.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.