 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.132.913 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H8N2O4Zn | |
| Molar mass | 309.59 g·mol−1 |
| Hazards | |
| GHS labelling: | |
 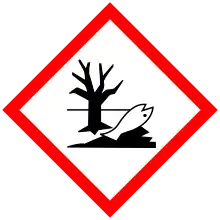 | |
| Warning | |
| H302, H315, H319, H335, H410 | |
| P261, P264, P270, P271, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P321, P330, P332+P313, P337+P313, P362, P391, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Zinc picolinate (or ZnPic) is the zinc salt of picolinic acid which has the molecular formula Zn(C6H4O2N)2.[1]
Zinc picolinate has been used as a dietary zinc supplement.[2][3][4]
References
- ↑ Lumme P, Lundgren G, Mark W (1969). "The crystal structure of zinc picolinate tetrahydrate" (PDF). Acta Chemica Scandinavica. 23: 3011–3022. doi:10.3891/acta.chem.scand.23-3011.
- ↑ Walsh CT, Sandstead HH, Prasad AS, Newberne PM, Fraker PJ (June 1994). "Zinc: health effects and research priorities for the 1990s". Environmental Health Perspectives. 102 (Suppl 2): 5–46. doi:10.1289/ehp.941025. PMC 1567081. PMID 7925188.
- ↑ Sakai F, Yoshida S, Endo S, Tomita H (2002). "Double-blind, placebo-controlled trial of zinc picolinate for taste disorders". Acta Oto-Laryngologica. Supplementum. 122 (546): 129–33. doi:10.1080/00016480260046517. PMID 12132610. S2CID 23717414.
- ↑ Barrie SA, Wright JV, Pizzorno JE, Kutter E, Barron PC (June 1987). "Comparative absorption of zinc picolinate, zinc citrate and zinc gluconate in humans". Agents and Actions. 21 (1–2): 223–8. doi:10.1007/BF01974946. PMID 3630857. S2CID 23567370.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.