The von Braun amide degradation is the chemical reaction of a monosubstituted amide with phosphorus pentachloride or thionyl chloride to give a nitrile and an organohalide.[1] It is named after Julius Jacob von Braun, who first reported the reaction.[2][3]
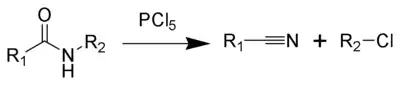
The von Braun amide degradation
Reaction mechanism
The secondary amide 1 reacts via its enolized form with phosphorus pentachloride to form the oxonium ion 2. This produces a chloride ion which deprotonates the oxonium ion to form and imine 3 and hydrogen chloride. These then react with one another to form an amine, with loss of the phosphorus chloride residue. The β-chloroimine 4 is unstable and undergoes internal elimination to a form a nitrilium cation 5 which is cleaved by attack by chloride to form a nitrile 6a and a haloalkane 6b.

See also
References
- ↑ von Pechmann, Hans (1900). "Ueber die Spaltung des Benzenylmethylimidchlorids". Berichte der deutschen chemischen Gesellschaft. 33 (1): 611–612. doi:10.1002/cber.19000330199.
- ↑ von Braun, J. (1904). "Ueber 1.5-Dibrompentan". Berichte der deutschen chemischen Gesellschaft. 37 (3): 3210–3213. doi:10.1002/cber.190403703118.
- ↑ Phillips, B.A.; Fodor, G.; Gal, J.; Letourneau, F.; Ryan, J.J. (1973). "Mechanism of the von Braun amide degradations with carbonyl bromide or phosphorus pentabromide". Tetrahedron. 29 (21): 3309–3327. doi:10.1016/S0040-4020(01)93483-0.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.