 | |
| Names | |
|---|---|
| Other names
Platinum--uranium (3/1) | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| UPt3 | |
| Molar mass | 823.3 g/mol[1] |
| Density | 19.3 g/cm3 |
| Melting point | 1700°C[2] |
| Structure | |
| see text | |
| P63/mmc | |
| Thermochemistry | |
Std molar entropy (S⦵298) |
-111 J·mol−1·K−1[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
UPt3 is an inorganic binary intermetallic crystalline compound of platinum and uranium.[1]
Production
It can be syntetized in the following ways:[3]
- as an intermetallic compound, by direct fusion of pure components according to stoichiometric calculations:
- by reduction of uranium dioxide with hydrogen in the presence of platinum:
Physical properties
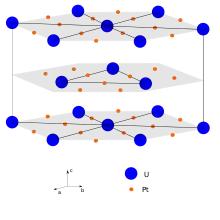
UPt3 forms crystals of hexagonal symmetry (some studies hypothesize a trigonal structure instead[4]), space group P63/mmc,[5] cell parameters a = 0.5766 nm and c = 0.4898 nm (c should be understood as distance from planes), with a structure similar to nisnite (Ni3Sn) and MgCd3.[6][7]
The compound congruently melts at 1700 °C.[2] The enthalpy of formation of the compound is -111 kJ/mol.[3]
At temperatures below 1 K it becomes superconducting, thought to be due to the presence of heavy fermions (the uranium atoms).[8][9]
References
- 1 2 PubChem. "Platinum--uranium (3/1)". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-10-18.
- 1 2 Lyakishev, N.P., ed. (2001). Диаграммы состояния двойных металлических систем [State diagrams of binary metal systems]. Mechanical Engineering (in Russian). Vol. 3, book 3. Moscow. p. 448. ISBN 5-217-02932-3.
{{cite book}}: CS1 maint: location missing publisher (link) - 1 2 3 Kleykamp, Heiko (1991). "Thermodynamics of the uranium-platinum metals systems" (PDF). Pure and Applied Chemistry. Vol. 63, no. 10. pp. 1401–1408. doi:10.1351/pac199163101401. Archived from the original on 14 February 2015. Retrieved 2022-10-17.
- ↑ Walko, D. A.; Hong, J.-I.; Chandrasekhar Rao, T. V. (2001-01-16). "Crystal structure assignment for the heavy-fermion superconductor UPt3". Physical Review B. Vol. 63, no. 5. p. 054522. doi:10.1103/PhysRevB.63.054522. Retrieved 2022-10-18.
- ↑ Sumita, Shuntaro; Yanase, Youichi (2018-04-13). "Unconventional superconducting gap structure protected by space group symmetry". Physical Review B. 97 (13): 134512. arXiv:1801.03293. Bibcode:2018PhRvB..97m4512S. doi:10.1103/PhysRevB.97.134512. S2CID 119100443.
- ↑ Predel (1998). "Pt-U (Platinum-Uranium)". Ni-Np – Pt-Zr. Landolt-Börnstein - Group IV Physical Chemistry. Springer-Verlag. pp. 1–2. doi:10.1007/10542753_2536. ISBN 3-540-61712-4.
- ↑ Ross, B. A. S.; Peterson, D. E. (1990-06-01). "The Pt-U (Platinum-Uranium) system". Bulletin of Alloy Phase Diagrams. Vol. 11, no. 3. pp. 240–243. doi:10.1007/BF03029291. Retrieved 2022-10-09.
- ↑ Gurtovoy, К. G.; Levitin, R. Z. (October 1987). "Магнетизм актинидов и их соединений" [Magnetism of actinides and their compounds] (PDF). Успехи физических наук (Advances in the Physical Sciences). Vol. 153, no. 2. Retrieved 2022-10-09.
- ↑ Mineev, V. P. (1994). "Superconductivity in UPt3". Annales de Physique. Vol. 19, no. 4. pp. 367–384. doi:10.1051/anphys:01994001904036700. Retrieved 2022-10-09.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.