3.png.webp) | |
| Names | |
|---|---|
| Preferred IUPAC name
Tris(2-methylphenyl)phosphane | |
| Other names
Tri(o-tolyl)phosphine; P(o-tol)3 | |
| Identifiers | |
| |
3D model (JSmol) |
|
| 661212 | |
| ChemSpider | |
| ECHA InfoCard | 100.025.631 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H21P | |
| Molar mass | 304.373 g·mol−1 |
| Appearance | White solid |
| Melting point | 124 °C (255 °F; 397 K) ±1 |
| Hazards | |
| GHS labelling: | |
 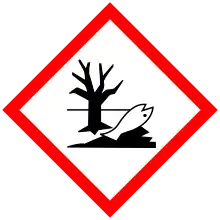 | |
| Warning | |
| H315, H319, H335, H410 | |
| P261, P264, P271, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P391, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Tris(o-tolyl)phosphine is an organophosphorus compound with the formula P(C6H4CH3)3. It is a white, water-insoluble solid that is soluble in organic solvents. In solution it slowly converts to the phosphine oxide. As a phosphine ligand, it has a wide cone angle of 194°. Consequently, it tends to cyclometalate when treated with metal halides and metal acetates. Complexes of this ligand are common in homogeneous catalysis.[1]

Structure of a Herrmann's catalyst, which is derived from tris(o-tolyl)phosphine.[2]
See also
References
- ↑ Richard F. W. Jackson; Manuel Perez-Gonzalez (2005). "Synthesis of N-(Tert-butoxycarbonyl)-β-iodoalanine Methyl Ester: A Useful Building Block in the Synthesis of Nonnatural α-amino Acids via Palladium Catalyzed Cross Coupling Reactions". Org. Synth. 81: 77. doi:10.15227/orgsyn.081.0077.
- ↑ Herrmann, W. A.; Brossmer, C.; Reisinger, C.-P.; Riermeier, T. H.; Öfele, K.; Beller, M. (1997). "Palladacycles: Efficient New Catalysts for the Heck Vinylation of Aryl Halides". Chemistry – A European Journal. 3 (8): 1357–1364. doi:10.1002/chem.19970030823.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.