 | |
| Names | |
|---|---|
| IUPAC name
Glycine sulfate (3:1) | |
| Other names
Glycine sulfate; TGS | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.007.414 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H17N3O10S | |
| Molar mass | 323.27 g·mol−1 |
| Appearance | White powder |
| Density | 1.69 g/cm3[1] |
| Structure | |
| Monoclinic | |
| P21[2] | |
a = 0.9417 nm, b = 1.2643 nm, c = 0.5735 nm α = 90°, β = 110°, γ = 90° | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Triglycine sulfate (TGS) is a chemical compound with a formula (NH2CH2COOH)3·H2SO4. The empirical formula of TGS does not represent the molecular structure, which contains protonated glycine moieties and sulfate ions. TGS with protons replaced by deuterium is called deuterated TGS or DTGS; alternatively, DTGS may refer to doped TGS. By doping the DTGS with the amino acid L-Alanine, the crystal properties are improved and the new material is called Deuterated L-Alanine doped Triglycine Sulfate (DLATGS or DLTGS). These crystals are pyroelectric and ferroelectric which allows their use as photodetector elements in infrared spectroscopy and night vision applications.[3] TGS detectors have also been used as the target in vidicon cathode ray imager tubes.
TGS has a critical point for the order parameter of polarization, at 322.5 K.[4]
Crystal structure and properties
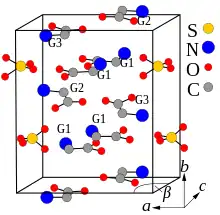
TGS crystals may be formed by evaporation of an aqueous solution of sulfuric acid and a greater than three-fold excess of glycine.[5] They belong to the polar space group P21 and therefore are pyroelectric and ferroelectric at room temperature, exhibiting spontaneous polarization along the b-axis ([010] direction). The Curie temperature of the ferroelectric transition is 49 °C for TGS and 62 °C for DTGS. The crystal structure consists of SO42−, 2(N+H3CH2COOH) (G1 and G2 in the crystal-structure diagram), and +NH3CH2COO− (G3) species held together by hydrogen bonds.[6] These bonds are easily broken by the polar molecules of water, which leads to the hygroscopicity of TGS – its crystals are easily etched by water. Along the b-axis, the G1-SO4 and G2-G3 layers are stacked alternately. The nearest two neighboring layers with identical chemical composition are rotated 180° around the b-axis against each other.[2][7] DTGS and DLATGS materials are derivatives of TGS which have better pyroelectric properties and give less detector noise as can be shown in the following table.
| Material | TGS | DTGS | DLATGS |
| Doping | - | D2O as a solvent | 20% wt. L-Alanine |
| Temperature of measurement (oC) | 25 | ||
| Curie temperature (oC) | 49 | 57-62 | 58-62 |
| Dielectric Constant at 1 kHz | 22-35 | 18-22.5 | 18-22 |
| Spontaneous Polarization (μC/cm2) | 2.75 | 2.6 | - |
| Coercive electric field (V/cm) | 165 V/cm | ||
| Inherent bias field (kV/cm) | 0.664-5 | 0.664-5 | 2-5 |
| Dielectric loss tan δ | ~1×10−3-10×10−3 | ||
| Figure of Merits (FOMs)
Fi =p (nC/cm2.oK) FV =p/ε´ (nC/cm2.oK) FD = p/√ε ′′ (nC/cm2.oK) |
16-45 0.5-1.14 0.4-121 |
25-70 1.4 - |
25 1.13 - |
| Volume Specific Heat (J/ cm3.oK) | 2.5 | 2.5 | 2.7 |
| Density (g/cm3) | 1.66 | 1.7 | 1.7 |
| AC Resistivity at 1 kHz (Ω.cm×1010) | 1.7 | 5 | 2.4 |
Typical performance of DLATGS detectors
The typical performance and pyroelectric properties of DLATGS detectors of 1.3 and 2.0 mm in diameter of the element size are shown in the table below.
| Element size (mm) | Vout at
1 kHz |
Voltage responsivity
V/W at 1 kHz |
Vn at 1 kHz
(1 Hz BW) |
D* at 1 kHz
Detectivity (cmHz1/2/W) |
C (pF) | tan δ | NEP
(W/√Hz) | |
| 1.3 | Typical | 3.20E-5 | 50 | 3.00E-8 Maximum | 2.70E+8 | 10.6 (at 20 μm) | 0.003 | 4.50E-10 |
| 2.0 | Typical | 3.20E-5 | 30 | 2.00E-8 Maximum | 3.50E+8 | 25 (at 25 μm) | 0.003 | 4.50E-10 |
References
- ↑ Kwan-Chi Kao (2004). Dielectric phenomena in solids: with emphasis on physical concepts of electronic processes. Academic Press. pp. 318–. ISBN 978-0-12-396561-5. Retrieved 12 May 2011.
- 1 2 3 Subramanian Balakumar and Hua C. Zeng (2000). "Water-assisted reconstruction on ferroelectric domain ends of triglycine sulfate (NH2CH2COOH)3·H2SO4 crystals". J. Mater. Chem. 10 (3): 651–656. doi:10.1039/A907937H.
- ↑ "Pyroelectric Detectors: Materials, Applications, and Working Principle" (PDF).
- ↑ Gonzalo, J. A. (1966-04-15). "Critical Behavior of Ferroelectric Triglycine Sulfate". Physical Review. 144 (2): 662–665. Bibcode:1966PhRv..144..662G. doi:10.1103/PhysRev.144.662.
- ↑ Pandya, G. R.; Vyas, D.D (1980). "Crystallization of glycine-sulfate". Journal of Crystal Growth. 5 (4): 870–872. Bibcode:1980JCrGr..50..870P. doi:10.1016/0022-0248(80)90150-5.
- ↑ Choudhury, Rajul Ranjan; Chitra, R. (2008). "Single crystal neutron diffraction study of triglycine sulphate revisited". Pramana. 71 (5): 911–915. Bibcode:2009Prama..71..911C. doi:10.1007/s12043-008-0199-5. S2CID 122953651.
- ↑ Wood, E.A.; Holden, A.N. (1957). "Monoclinic glycine sulfate: crystallographic data". Acta Crystallogr. 10 (2): 145–146. doi:10.1107/S0365110X57000481.
- ↑ D., Aggarwal, M. (2010). Pyroelectric materials for uncooled infrared detectors : processing, properties, and applications. National Aeronautics and Space Administration, Marshall Space Flight Center. OCLC 754804811.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ "DEVELOPMENT OF IMPROVED PYROELECTRIC DETECTORS" (PDF).
- ↑ "Pyroelectric materials" (PDF).
- ↑ Aravazhi, S; Jayavel, R; Subramanian, C (1997-10-15). "Growth and stability of pure and amino doped TGS crystals". Materials Chemistry and Physics. 50 (3): 233–237. doi:10.1016/S0254-0584(97)01939-1. ISSN 0254-0584.
- ↑ Aggarwal, M.D., Batra, A.K., Guggilla, P., Edwards, M.E., Penn, B.G. and Currie Jr, J.R. "Pyroelectric materials for uncooled infrared detectors: processing, properties, and applications" (PDF). NASA Technical Memorandum.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ https://ntrs.nasa.gov/api/citations/19720013777/downloads/19720013777.pdf
- ↑ Srinivasan, M. R. (1984-05-01). "Pyroelectric materials". Bulletin of Materials Science. 6 (2): 317–325. doi:10.1007/BF02743905. ISSN 0973-7669. S2CID 189911723.
- ↑ Company, Leonardo. "DLATGS Detectors" (PDF).
- ↑ Components, Laser. "D31 / LT31 Series Single Channel Voltage Mode Pyroelectric Detectors".