 | |
 | |
| Names | |
|---|---|
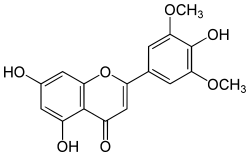
| IUPAC name
4′,5,7-Trihydroxy-3′,5′-dimethoxyflavone | |
| Systematic IUPAC name
5,7-Dihydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)-4H-1-benzopyran-4-one | |
| Other names
Tricetin 3′,5′-dimethyl ether | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C17H14O7 | |
| Molar mass | 330.29 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Tricin is a chemical compound. It is an O-methylated flavone, a type of flavonoid. It can be found in rice bran[1] and sugarcane.[2]
Glycosides
- Tricin 4'-glucoside (Tricin-4'-O-beta-D-glucopyranaoside, CAS number 71855-50-0)
- Tricin 5-glucoside (Tricin 5-O-beta-D-glucopyranoside, CAS number 32769-00-9)
- Tricin 7-O-glucoside (Tricin 7-O-beta-D-glucopyranoside, CAS number 32769-01-0)
Biosynthesis
The biosynthesis of flavones has not yet been elucidated in full; however, most of the mechanistic and enzymatic steps have been discovered and studied. In biosynthesizing tricin, there is first stepwise addition of malonyl CoA via the polyketide pathway and p-coumaroyl Coa via the phenylpropanoid pathway.[3] These additions are mediated by the sequential action of chalcone synthase and chalcone isomerase to yield naringenin chalcone and the flavanone, naringenin, respectively. CYP93G1 of the CYP450 superfamily in rice then desaturates naringenin into apigenin. After this step, it is proposed that flavonoid 3’5’-hydroxylase (F3’5’H) changes apigenin into tricetin.[4] Upon formation of tricetin, 3’-O-methyltransferase and 5’-O-methyltransferase adds methoxy groups to tricetin to form tricin.
Other compounds formed from tricin
Three flavonolignans derived from tricin have been isolated from oats Avena sativa.[5]
References
- ↑ The rice bran constituent tricin potently inhibits cyclooxygenase enzymes and interferes with intestinal carcinogenesis in ApcMin mice
- ↑ Alves, VG; Souza, AG; Chiavelli, LU; Ruiz, AL; Carvalho, JE; Pomini, AM; Silva, CC (2016). "Phenolic compounds and anticancer activity of commercial sugarcane cultivated in Brazil". An. Acad. Bras. Ciênc. 88 (3): 1201–9. doi:10.1590/0001-3765201620150349. PMID 27598841.
- ↑ Zhou, Jian-Min; Ibrahim, Ragai K. (2009). "Tricin—a potential multifunctional nutraceutical". Phytochemistry Reviews. 9 (3): 413–424. doi:10.1007/s11101-009-9161-5.
- ↑ Lam, PY; Zhu, FY; Chan, WL; Liu, H; Lo, C (2014). "Cytochrome P450 93G1 Is a Flavone Synthase II That Channels Flavanones to the Biosynthesis of Tricin O-Linked Conjugates in Rice". Plant Physiol. 165 (3): 1315–1327. doi:10.1104/pp.114.239723. PMC 4081339. PMID 24843076.
- ↑ Wenzig, Eva (2005). "Flavonolignans from Avena sativa". Journal of Natural Products. 68 (2): 289–292. doi:10.1021/np049636k. PMID 15730266.