 | |
| Names | |
|---|---|
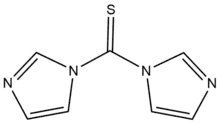
| Preferred IUPAC name
Di(1H-imidazol-1-yl)methanethione | |
| Other names
TCDI | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.025.622 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H6N4S | |
| Molar mass | 178.21 g·mol−1 |
| Melting point | 101 to 103 °C (214 to 217 °F; 374 to 376 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
1,1'-Thiocarbonyldiimidazole (TCDI) is a thiourea containing two imidazole rings.[1] It is the sulfur analog of the peptide coupling reagent carbonyldiimidazole (CDI).
Synthesis
TCDI is commercially available but can also be prepared via the reaction of thiophosgene with two equivalents of imidazole.[1]
Reactions
The imidazole groups on TCDI can be easily displaced, allowing it to act as a safer alternative to thiophosgene. This behaviour has been used in the Corey–Winter olefin synthesis.[2] It may also replace carbonothioyl species (RC(S)Cl) in the Barton–McCombie deoxygenation. Other uses include the synthesis of thioamides and thiocarbamates. Like the analogous CDI, it may be used for peptide coupling.[3]
References
- 1 2 Adrian, L. Schwan; Jeffrey, H. Byers (15 March 2007). "1,1′‐Thiocarbonyldiimidazole". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/9780470842898.rt094.pub2.
- ↑ Corey, E. J.; Winter, Roland A. E. (September 1963). "A New, Stereospecific Olefin Synthesis from 1,2-Diols". Journal of the American Chemical Society. 85 (17): 2677–2678. doi:10.1021/ja00900a043.
- ↑ Esser, Franz; Roos, Otto (June 1978). "N-Terminal Cyclization of Peptides with N,N′-Carbonyldiimidazole orN,N′-Thiocarbonyldiimidazole". Angewandte Chemie International Edition in English. 17 (6): 467–468. doi:10.1002/anie.197804671.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.