 | |
| Names | |
|---|---|
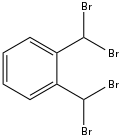
| Preferred IUPAC name
1,2-Bis(dibromomethyl)benzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.032.873 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H6Br4 | |
| Molar mass | 421.752 g·mol−1 |
| Appearance | off white solid |
| Melting point | 115–116 °C (239–241 °F; 388–389 K) |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H314, H335, H400 | |
| P260, P261, P264, P271, P273, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P363, P391, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
α,α,α',α'-Tetrabromo-o-xylene is an organobromine compound with the formula C6H4(CHBr2)2. Three isomers of α,α,α',α'-Tetrabromoxylene exist, but the ortho derivative is most widely studied. It is an off-white solid. The compound is prepared by the photochemical reaction of o-xylene with elemental bromine:[1]
- C6H4(CH3)2 + 4 Br2 → C6H4(CHBr2)2 + 4 HBr
Reaction of α,α,α',α'-tetrabromo-o-xylene with sodium iodide affords α,α'-dibromo-o-xylylene, which can be trapped with dienophiles to give naphthylene. In the absence of trapping agents, the xylylene relaxes to α,α'-dibromobenzocyclobutane:[2]
- C6H4(CHBr2)2 + 2 NaI → C6H4(=CHBr)2 + 2 NaBr + I2
- C6H4(=CHBr)2 → C6H4(CHBr)2
Cycloadditions of these xylylenes provides a pathway to acenes.[3]
References
- ↑ Bill, J. C.; Tarbell, D. S. (1954). "o-Phthalaldehyde". Organic Syntheses. 34: 82. doi:10.15227/orgsyn.034.0082.
- ↑ Cava, M. P.; Deana, A. A.; Muth, K. (1959). "Condensed Cyclobutane Aromatic Compounds. VIII. The Mechanism of Formation of 1,2-Dibromobenzocyclobutene; A New Diels-Alder Synthesis". Journal of the American Chemical Society. 81 (24): 6458–6460. doi:10.1021/ja01533a032.
- ↑ Paddon-Row, Michael N.; Patney, Harish K. (1986). "An Efficient Synthetic Strategy for Naphthalene Annellation of Norbornenylogous Systems". Synthesis. 1986 (4): 328–330. doi:10.1055/s-1986-31603.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.