 | |
| Names | |
|---|---|
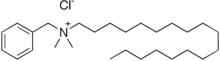
| Preferred IUPAC name
N-Benzyl-N,N-dimethyloctadecan-1-aminium chloride | |
| Other names
Dimethylbenzyloctadecylammonium chloride; Benzyldimethyloctadecylammonium chloride; Benzyldimethylstearylammonium chloride; Benzylstearyldimethylammonium chloride; N,N-dimethyl-n-octadecylbenzenemethanaminium chloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.004.117 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C27H50ClN | |
| Molar mass | 424.15 g·mol−1 |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
1250 mg/kg (oral, rat) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Stearalkonium chloride is a type of benzalkonium chloride which is used as an anti-static agent, a surfactant and an antimicrobial.[1] It is an ingredient in some cosmetics and hair care products, particularly conditioners.[2] It was originally designed by the fabric industry for use as a fabric softener.
Toxicology studies have determined that stearalkonium chloride is safe and non-toxic at the concentrations typically used in cosmetic products (0.1 to 5%).[3] At higher concentrations (25% solution), it has been shown to cause minor skin and eye irritation in animals.[3]
See also
- Benzalkonium chloride – Surfactant and antiseptic agent
- Polyaminopropyl biguanide – chemical compound, an alternative preservative for contact lens solutions
- Ethylenediaminetetraacetic acid – chemical compound used for industrial and chemical purpose
- Triclosan – Antimicrobial agent
- Thiomersal – Organomercury antiseptic and antifungal agent
References
- 1 2 Stearyl dimethyl benzyl ammonium chloride, chemicalland21.com
- ↑ Stearalkonium chloride in the Consumer Product Information Database
- 1 2 "Final Report on the Safety Assessment of Stearalkonium Chloride". International Journal of Toxicology. 1 (2): 57–69. 1982. doi:10.3109/10915818209013147. S2CID 208507695.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.