| Staphylococcus schleiferi | |
|---|---|
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Bacillota |
| Class: | Bacilli |
| Order: | Bacillales |
| Family: | Staphylococcaceae |
| Genus: | Staphylococcus |
| Species: | S. schleiferi |
| Binomial name | |
| Staphylococcus schleiferi Freney et al. 1988 | |
| Subspecies | |
| |
Staphylococcus schleiferi is a Gram-positive, cocci-shaped bacterium of the family Staphylococcaceae.[1] It is facultatively anaerobic, coagulase-variable, and can be readily cultured on blood agar where the bacterium tends to form opaque, non-pigmented colonies and beta (β) hemolysis.[2] There exists two subspecies under the species S. schleiferi: Staphylococcus schleiferi subsp. schleiferi (coagulase negative) and Staphylococcus schleiferi subsp. coagulans (coagulase positive).[3]
Staphylococcus schleiferi is commonly recognized as a veterinary pathogen affecting household pets, but has not been identified as a disease causing organism in large animals.[4][5] S. schleiferi has been identified as a causative agent of conditions of Pyoderma, Otitis Externa, and Otitis media in both dogs and cats;[4] although more commonly causing inflammatory conditions in dogs than in cats.[6] Human infections have been described in some case reports, resulting in certain disease conditions including: surgical site infections, pediatric meningitis, endocarditis, and intravascular device-related bacteremia.[7] Although both companion animals and humans can acquire disease from this organism, its zoonotic potential is not well understood. Antimicrobial therapy has been generally successful in treatment of infections, however, resistance to beta-lactam antibiotics have been reported, resulting in persistent infections for both humans and veterinary species.[8]
Since its first description in 1988, little has been reported regarding the pathogenicity and virulence of Staphylococcus schleiferi.[9] However, similarities with infections caused by Staphylococcus aureus suggest that the two species may also share similar determinants of virulence.[10] Virulence factors associated with S. schleiferi have been identified to include the production of fatty acid modifying enzyme (FAME), biofilms, penicillin-binding protein 2a (PBP2a), as well as various enterotoxins and exoenzymes.[11][12][13][14][15]
Staphylococcus schleiferi is differentiated from other Staphylococcal species based on their coagulation reaction, but because there is a coagulase positive and a coagulase negative subspecies of S. schleiferi, additional biochemical tests are required.[16] These tests are often not done clinically as treatment is based on susceptibility testing and location of the infection.[17]
Microbiology
History and taxonomy
In 1988, Freney et al. isolated two previously unidentified Staphylococcus species from human clinical specimens: S. schleiferi and S. lugdunensis.[1] The former species was named schleiferi in honor of German microbiologist Karl Heinz Schleifer, to mark his significant contributions to the taxonomy of gram-positive bacteria.[18] Later in 1990, a coagulase-positive subtype was isolated from dogs and cats by Igimi et al.[3] This led to the classification of Staphylococcus schleiferi into two distinct subspecies, the coagulase-negative S. schleiferi schleiferi and the coagulase-positive S. schleiferi coagulans.[19] Both S. schleiferi subspecies have since been reported to be linked to an array of infections in humans and companion animals.[3][20]
Cellular morphology
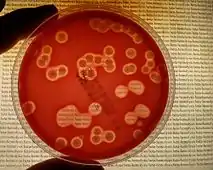
Staphylococcus schleiferi is a facultatively anaerobic, coagulase-variable, Gram-positive cocci organism.[1] It is nonmotile and nonspore-forming.[21] When cultured on 5% sheep blood agar, isolates of S. schleiferi form circular, opaque, non-pigmented colonies of approximately 0.8 to 1.0μm in diameter.[2][21] A complete (β) hemolysis can be seen on blood agar as well. On a Gram stain, S. schleiferi appears as individuals, pairs, small clusters, or chains of 3 to 7 cells.[21]
Biochemistry and identification
Staphylococcus schleiferi can be readily identified using matrix-assisted laser desorption ionization time of flight (MALDI-TOF), although differentiation to the subspecies level often requires biochemical testing with tube coagulase and urease reactions. S. schleiferi subspecies schleiferi tests negative for tube coagulase and urease, whereas S. schleiferi subspecies coagulans tests positive for tube coagulase and urease.[22] Commercial identification systems often recommend the use of additional biochemical tests to further confirm an identification of S. schleiferi.
Differentiation from Staphylococcus aureus

Staphylococcus schleiferi can often be mistaken for Staphylococcus aureus as both staphylococcal species produce heat-stable DNase and clumping factor. Moreover, colonies of S. aureus appear morphologically similar when grown on blood agar.[23] Many have even suggested that there is an underestimation of reported S. schleiferi infections due to false identifications of S. schleiferi as S. aureus.[23][24] Multiple biochemical tests can be performed to differentiate these related Staphylococcus species, although some analyses, such as the tube coagulase test, are not performed in routine laboratory procedures. Sugar fermentation tests, for instance, can be performed as S. schleiferi does not acidify maltose, mannitol, or sucrose, as opposed to S. aureus.[25] S. schleiferi also demonstrates pyrrolidonyl arlamidase (PYR) activity, whereas S. aureus tests negative for PYR enzymatic activity.[26] S. schleiferi can also be discerned from S. aureus by production of a different thermonuclease that lacks pigmentation.[27] In contrast to S. schleiferi which produces β-hemolysin and consequently exhibits a complete (β) hemolysis, strains of S. aureus can produce double-zone (α + β) hemolysis.[28]
Differentiation from Staphylococcus lugdunensis
Although Staphylococcus schleiferi and Staphylococcus lugdunensis both demonstrate PYR activity and production of clumping factor, these staphylococcal species can be differentiated in their different hemolytic activities on blood agar. While S. schleiferi presents a complete (β) hemolysis, S. lugdunensis produces a double-zone (α + β) hemolysis.[29] S. schleiferi is also capable of adherence to glass, while S. lugdunensis fails to adhere to glass.[29]
Epidemiology
Prevalence
Staphylococcus schleiferi is recognized as commensal microflora on the skin of humans and animals like many other Staphylococci species.[30] It is more commonly recognized as a veterinary pathogen affecting household pets; in particular, S. schleiferi has been isolated from healthy dogs as well as dogs with skin and ear infections.[5]
Staphylococcus schleiferi is less commonly associated with human infection, but can be nosocomial acquired. A study performed at a tertiary care centre in Northern Spain found that out of 28 patients documented with S. schleiferi infection, 89.3% were men.[31] Over half of the patients that were infected also had some degree of immunosuppression, namely malignant neoplasm. Most infections were also related to wound-infection (mainly surgical-site infections) - however, infection-related mortality was low.[31]
Geographical distribution
Staphylococcus schleiferi has a worldwide distribution. This opportunistic pathogen has been isolated from dogs with pyoderma and otitis externa in Korea,[32] Japan,[33] France,[19] Italy,[34] and the West Indies.[35] . S. schleiferi was the second most prevalent species present in samples collected from dogs with pyoderma and otitis externa in Korea.[32] It has also been isolated from 36 patients in northern Spain from 1993-1999.[31]
Staphylococcus schleiferi was isolated from a multitude of pinniped species and penguins in the Antarctic and Scotland.[36]
Antimicrobial resistance
Methicillin-resistance
Methicillin-resistant staphylococci is a growing public health concern, with systemic use of antibiotics becoming more common. Systemic antibiotic use has been associated with the development of infections with MR staphylococci.[37] Increased prevalence of methicillin-resistant staphylococci has been reported in specialty dermatology practices in the United States[8] and in Canada.[38] A study performed at University of Pennsylvania School of Veterinary Medicine found that 40% of S. schleiferi were resistant to methicillin.[8] At the University of Tennessee, 46.6% of the S. schleiferi isolated were resistant to oxacillin.[39] Seven strains of methicillin-resistant S. schleiferi (MRSS) were also isolated from dogs presenting with pyoderma and otitis externa in Korea.[32]
Compared to other MR staphylococci, MRSS maintained the most favourable susceptibility profile.[8] However, to avoid selecting for resistant strains, culture and susceptibility testing is crucial prior to starting a course of treatment.
Fluoroquinolone resistance
Eight isolates of S. schleiferi from canine patients were tested against 23 antimicrobial agents. 62.5% showed resistance to multiple fluoroquinolones.[34] A similar study found only 40% of S. schleiferi isolates to be susceptible to all 16 fluoroquinolones tested against it.[40]
Although the current antimicrobials commonly used for treatment of S. schleiferi caused infections experimentally show susceptibility, the changes in temporal trends and different resistance patterns for S. schleiferi emphasize the importance of antimicrobial susceptibility testing to choose the most appropriate treatment of infections.[34]
Zoonotic potential
Staphylococcus species were initially thought to be host-specific pathogens, however, human strains of S. intermedius, S. schleiferi, and S. aureus have been isolated from animal reservoirs, indicating their multi-host potential.[41][42] S. schleiferi is a known canine skin pathogen, causing pyoderma, otitis externa, and otitis media in healthy dogs with no pre-existing risk factors;[43][44] and has also been reported to infect humans, causing a multitude of nosocomial infections such as endocarditis, osteomyelitis, septic arthritis, UTIs, and wound infections.[4][45] It is unknown what role zoonotic transmission has in human disease acquisition associated with S. schleiferi, however, there is growing evidence of zoonoses occurring with other related Staphylococcus species.
Evidence of zoonosis in Staphylococcus species
Staphylococcus aureus
Methicillin-Resistant S. aureus (MRSA) has been a growing public health concern, with increases in infection prevalence in individuals with no apparent risk factors.[46] Both zoonotic and reverse zoonotic transmission have been reported for MRSA, indicating the ability for the bacteria to accumulate on animal reservoirs, and to reinfect humans.[47]
Staphylococcus intermedius
S. intermedius is a common commensal of dogs and cats, though rarely causes infections in humans. However, infections have been found in people with relation to household pets, resulting in a report of postoperative sinus infection,[48] otitis externa, bite wounds, catheter related injuries, and surgery.[49] Owners of dogs affected by deep pyoderma carried multiple anti-microbial resistant strains of S. intermedius which is thought to be transferred between the canine and human pathogenic staphylococci.[42]
Staphylococcus pseudintermedius
S. pseudintermedius is considered a novel species of Staphylococcus, and is a commensal organism found on the skin and mucous membranes of dogs.[50] Transmission from canines is suspected to cause skin and soft tissue infections in people.[51] S. pseudintermedius was also isolated from skin breaks of child with eczema following licking from the family dog.[52]
Staphylococcus schleiferi
Although there is little evidence outlining the incidence of zoonotic transmission, the increasing recognition of Methicillin-Resistant isolates of S. schleiferi may have importance to public health, as there is already concern regarding possible transfer of resistance genes from canine to human staphylococci species.[8][42]
Virulence
The mechanisms that Staphylococcus schleiferi employ to carry out its virulence are not well elucidated however, similarities between infections of S. schleiferi and other Staphylococcus spp. such as Staphylococcus aureus suggest that these species also share similar determinants of virulence.[10]
Fatty acid modifying enzyme (FAME) and lipase
The production of fatty acid modifying enzyme (FAME) and lipase has been identified as potential virulence factors in various Staphylococcus species including S. schleiferi.[11] The production of both FAME and lipase assists the organism in circumventing host defenses such as bactericidal lipids, thus allowing its persistence and survival within host tissues.[11] FAME produced by Staphylococcus inhibits bactericidal fatty acids which are a first line of defense against invading organisms during abscess formation.[53] The production of lipase also prevents glycerides from inhibiting the activity of FAME, thus expression of both enzymes is thought to be required for the survival of Staphylococcus within abscesses.[11]
Biofilm
Many Staphylococcus spp. possess the capacity to produce biofilm: a polysaccharide matrix which contributes to the organism's ability to resist antimicrobial therapeutics, evade the host's immune system, and survive on inanimate surfaces.[54] Methicillin-resistant strains of S. schleiferi have been found to possess this ability to produce biofilm which limits access to the organism by antimicrobial therapeutic agents and is thought to also provide protection against host defense cationic antimicrobial peptides.[12] Additionally, S. schleiferi has been shown to express cell wall-anchored fibronectin-binding proteins which may play a role in its pathogenesis by facilitating adherence to host cells and proteins, as well as to medical devices which can become important sources of nosocomial infection.[10][55]
Antibiotic resistance
Antibiotic resistance plays a critical role in the development and persistence of infection and although is not considered to be a virulence factor alone, may act as a virulence-like factor in unique circumstances by facilitating the colonization of opportunistic pathogens such as Staphylococcus schleiferi, allowing them greater opportunity to cause disease such as in nosocomial infections.[56]
Methicillin resistance within the Staphylococcus species is facilitated by the spread of the mecA gene which codes for penicillin-binding protein 2a (PBP2a).[57] The mecA gene is carried by a mobile genetic element called the staphylococcal cassette chromosome mec (SCCmec) which is thought to promote spread between different species.[58] The presence of the mecA gene, expression of PBP2a, and methicillin resistance has been reported in S. schleiferi isolates.[13] Penicillin-binding proteins are critical in the crosslinking reaction required for the synthesis of peptidoglycan and are the targets of beta-lactam antibiotics. However, PBP2a encoded by the mecA gene have reduced affinity for, and thus is not inhibited by, most beta-lactam antibiotics thus conferring resistance against most beta-lactam antibiotics.[59]
Staphylococcus schleiferi has also shown resistance to fluoroquinolones, including second and third generation fluoroquinolones, but may retain susceptibility to fourth generation fluoroquinolones.[40] This resistance was associated with changes in the gyrA gene which encodes for DNA gyrase subunit A, resulting in less susceptibility of the enzyme to fluoroquinolones.[40]
Enterotoxins and exoenzymes
Many Staphylococcal species produce enterotoxins which have known pyrogenic and emetic effects.[60] PCR analysis has detected the presence of enterotoxin producing genes sed and ELISA methods have shown the production of the corresponding staphylococcal enterotoxin SED by S. schleiferi.[14] SED is thought to be one of the most common enterotoxins produced by Staphylococcus spp. associated with food poisoning.[60] S. schleiferi also produce staphylococcal enterotoxins SEA, SEB, SEC and toxic-shock syndrome toxin (TSST-1).[61] Enterotoxins SEA and SEB are known emetics in primates and, with TSST-1, cause Toxic Shock Syndrome with acute intoxication.[62]
Staphylococcus schleiferi also possess the ability to produce numerous exoenzymes such as alpha and delta toxins, DNase, lipase, esterase, and protease which may also contribute to its virulence or serve as aggressins.[15] A beta-like toxin similar in structure and functionality to the beta-toxin of S. aureus has also been described in S. schleiferi.[63]
Disease
Diseases of dogs and cats
Staphylococcus schleiferi is most commonly identified as a pathogenic bacteria of companion animals (primarily dogs and cats).[6] Staphylococcus schleiferi rarely causes disease in cats, and it is more commonly associated with inflammatory conditions of dogs.[64] Staphylococcus schleiferi can be involved in conditions of Pyoderma, Otitis Externa, and Otitis media in both dogs and cats.[4]
Pyoderma
Staphylococcus schleiferi is one of the causative agents in pyoderma of dogs and cats. Pyoderma is a skin infection characterized by the presence of purulent discharge (pus).[65] Dogs are most commonly affected by this skin infection which may be caused by a bacterial infection or sometimes, but less commonly, a fungal infection. Staphylococcus schleiferi along with Staphylococcus aureus and Staphylococcus pseudintermedius are the most common bacteria that cause pyoderma in dogs.[65] Staphylococcus schleiferi is more commonly found in cases of pyoderma with dogs suffering from reoccurring pyoderma who have already undergone antimicrobial treatment. Staphylococcus pseudintermedius and Staphylococcus aureus are more commonly found to be the cause of pyoderma in dogs who are experiencing the infection for the first time.[66] The symptoms of pyoderma include: pruritus (severe itchiness); dermatitis (general skin irritation); alopecia (hair loss); scaling/scabbing; and bloody and/or purulent discharge.[67] When treating a dog with pyoderma related to Staphylococcus schleiferi, it is important to avoid administering methicillin and other penicillins, as there is increasing resistance to these antimicrobial therapies.[34]
Otitis externa
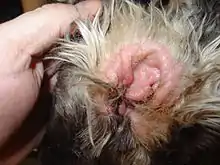
Otitis Externa is an inflammatory condition of the outer ear canal that affects many species, including canids. Staphylococcus schleiferi has been identified as one of the organisms which contributes to Otitis Externa in dogs and less commonly in cats.[68] Otitis Externa is the most common disorder of the ear canal of dogs.[69] Clinical signs of Otitis Externa include: head shaking, alopecia (hair loss), erythema (reddening of the skin), and pruritus (itchiness).[70] There appears to be a higher incidence of Otitis Externa in young dogs (1–5 years of age) as compared to older dogs (>5 years of age).[71] There is also disposition to Otitis Externa in certain breeds, including: Cocker Spaniels, Golden Retrievers, and West Highland White Terriers.[70] Treatment of Otitis Externa depends on the cause. There are multiple organisms that may cause this inflammation and infection of the ear canal. Treatment plans should be decided based on bacterial identification and susceptibility profiles.[70]
Otitis media
Staphylococcus schleiferi has been identified as a contributor to Otitis media in dogs and less commonly cats.[4] Otitis media is a condition of inflammation of the middle ear canal. Otitis Media is concurrently present in many of the cases of dogs diagnosed with Otitis Externa.[72] If Otitis Media is not diagnosed and treated, it can lead to Otitis Externa. Signs of Otitis Media include: head shaking, vestibular signs (head tilt), and scratching of the effected ear.[73] Diagnosis of Otitis Media is more challenging than with Otitis Externa because access to the middle ear canal can be challenging. Following diagnosis, bacterial identification is required and susceptibility testing on the bacteria is warranted to guide the microbial treatment plan. Surgery is a treatment option when antimicrobial treatment fails to resolve the clinical signs associated with Otitis Media.[72]
Diseases of humans
Staphylococcus schleiferi has rarely been described as a human pathogen, but there are some case reports and case series reports that describe the correlation between isolation of Staphylococcus schleiferi and surgical site and wound infections.[7]
Staphylococcus schleiferi has been described as the causative agent of surgical site and wound infections; pediatric meningitis; endocarditis; and intravascular device-related bacteremia in case reports and case series reports:
Surgical site and wound infections
Staphylococcus schleiferi has been described in a clinical case series report as causing infections at surgical sites post-operatively.[24][31]
Pediatric meningitis

Meningitis refers to inflammation of the meninges. Staphylococcus schleiferi has been identified as the causative agent of meningitis in a child (6 years old) and an infant (2 months old) in case reports.[74][75]
Endocarditis
Endocarditis refers to inflammation of the endocardium of the heart. Staphylococcus schleiferi was isolated as the cause of endocarditis of a prosthetic valve in a case report involving a 78-year-old man.[76]
Intravascular device-related bacteremia
In a case report describing a 55-year-old female who had recently had a left ventricular assist device placed, Staphylococcus schleiferi was identified as the causative agent of Bacteremia. A second case was described involving a 58-year-old male who had undergone a liver transplant and subsequently developed Staphylococcus schleiferi aortic valve endocarditis.[77]
Diagnosis
A swab collected from the area of interest is regularly taken because Staphylococcus schleiferi is often associated with superficial infections of the skin for people and skin or ears for animals.[78][79] Sample collection does depend on the site of interest and so an appropriate specimen is obtained based on the area of infection, such as a cystocentesis for urinary tract infections.[80][79] The initial step of gram staining assists in distinguishing the characteristic gram-positive cocci in clusters for Staphylococcus species.[78][81] It is then cultured on blood agar as non-pigmented round colonies that are beta-hemolytic meaning there is complete clearing of the red blood cells.[16]
Staphylococcal species are typically differentiated based on their coagulation reaction but because Staphylococcus schleiferi is a coagulase variable species, meaning it can appear positive or negative on coagulase testing depending on the subtype, additional biochemical tests are needed to be performed.[81][16] Further testing may include polymerase chain reaction (PCR) and matrix-assisted laser desorption ionization time of flight mass spectrometry (MALDI-TOF MS).[79][81] PCR amplifies DNA for identification whereas MALDI-TOF uses both the mass and charge of molecules to acquire a unique peptide mass fingerprint (PMF).[82] The PMF is matched to a database of known microbial isolates, but this database is a limitation as the database must contain the PMF for the tested organism.[83] MALDI-TOF MS has been reliable for distinguishing S. schleiferi from other Staphylooccus species but not for identifying subspecies like schleiferi and coagulans.[84][85] Clinically, subspecies identification is commonly not done as treatment is based on susceptibility testing and location of the infection.[17]
References
- 1 2 3 Fleurette J, Bès M, Brun Y, Freney J, Forey F, Coulet M, et al. (February 1989). "Clinical isolates of Staphylococcus lugdunensis and S. schleiferi: bacteriological characteristics and susceptibility to antimicrobial agents". Research in Microbiology. 140 (2): 107–18. doi:10.1016/0923-2508(89)90044-2. PMID 2799061.
- 1 2 Huse HK, Miller SA, Chandrasekaran S, Hindler JA, Lawhon SD, Bemis DA, et al. (February 2018). "mecA-Mediated Oxacillin Resistance in Staphylococcus schleiferi". Journal of Clinical Microbiology. 56 (2). doi:10.1128/jcm.01653-17. PMC 5786728. PMID 29187565.
- 1 2 3 Igimi S, Takahashi E, Mitsuoka T (October 1990). "Staphylococcus schleiferi subsp. coagulans subsp. nov., isolated from the external auditory meatus of dogs with external ear otitis". International Journal of Systematic Bacteriology. 40 (4): 409–11. doi:10.1099/00207713-40-4-409. PMID 2275856.
- 1 2 3 4 5 Sykes JE, Nagle TM, White SD (2014-01-01). "Chapter 84 - Pyoderma, Otitis Externa, and Otitis Media". In Sykes JE (ed.). Canine and Feline Infectious Diseases. Saint Louis: W.B. Saunders. pp. 800–813. ISBN 978-1-4377-0795-3.
- 1 2 May ER, Kinyon JM, Noxon JO (December 2012). "Nasal carriage of Staphylococcus schleiferi from healthy dogs and dogs with otitis, pyoderma or both". Veterinary Microbiology. 160 (3–4): 443–8. doi:10.1016/j.vetmic.2012.06.020. PMID 22771206.
- 1 2 Malik S, Peng H, Barton MD (2005–2012). "Antibiotic resistance in staphylococci associated with cats and dogs". Journal of Applied Microbiology. 99 (6): 1283–93. doi:10.1111/j.1365-2672.2005.02699.x. PMID 16313400. S2CID 40949486.
- 1 2 Davis MF, Cain CL, Brazil AM, Rankin SC (2013). "Two coagulase-negative staphylococci emerging as potential zoonotic pathogens: wolves in sheep's clothing?". Frontiers in Microbiology. 4: 123. doi:10.3389/fmicb.2013.00123. PMC 3654208. PMID 23720657.
- 1 2 3 4 5 Morris DO, Rook KA, Shofer FS, Rankin SC (October 2006). "Screening of Staphylococcus aureus, Staphylococcus intermedius, and Staphylococcus schleiferi isolates obtained from small companion animals for antimicrobial resistance: a retrospective review of 749 isolates (2003-04)". Veterinary Dermatology. 17 (5): 332–7. doi:10.1111/j.1365-3164.2006.00536.x. PMID 16961819.
- ↑ Calvo J, Hernández JL, Fariñas MC, García-Palomo D, Agüero J (October 2000). "Osteomyelitis caused by Staphylococcus schleiferi and evidence of misidentification of this Staphylococcus species by an automated bacterial identification system". Journal of Clinical Microbiology. 38 (10): 3887–9. doi:10.1128/JCM.38.10.3887-3889.2000. PMC 87502. PMID 11015429.
- 1 2 3 Peacock SJ, Lina G, Etienne J, Foster TJ (August 1999). "Staphylococcus schleiferi subsp. schleiferi expresses a fibronectin-binding protein". Infection and Immunity. 67 (8): 4272–5. doi:10.1128/iai.67.8.4272-4275.1999. PMC 96737. PMID 10417204.
- 1 2 3 4 Long JP, Hart J, Albers W, Kapral FA (October 1992). "The production of fatty acid modifying enzyme (FAME) and lipase by various staphylococcal species". Journal of Medical Microbiology. 37 (4): 232–4. doi:10.1099/00222615-37-4-232. PMID 1404319.
- 1 2 Lee GY, Lee HH, Hwang SY, Hong J, Lyoo KS, Yang SJ (March 2019). "Staphylococcus schleiferi from canine otitis externa: antimicrobial resistance profiles and virulence factors associated with skin infection". Journal of Veterinary Science. 20 (2): e6. doi:10.4142/jvs.2019.20.e6. PMC 6441802. PMID 30944529.
- 1 2 Kania SA, Williamson NL, Frank LA, Wilkes RP, Jones RD, Bemis DA (September 2004). "Methicillin resistance of staphylococci isolated from the skin of dogs with pyoderma". American Journal of Veterinary Research. 65 (9): 1265–8. doi:10.2460/ajvr.2004.65.1265. PMID 15478775.
- 1 2 Zigo F, Vasiľ M, Elečko J, Lapin M, Farkašova Z (2014-05-07). "Production of enterotoxins of Staphylococcus spp. isolated from samples of sheep milk". Potravinarstvo. 8 (1): 92–96. doi:10.5219/361. ISSN 1337-0960.
- 1 2 Lambe DW, Ferguson KP, Keplinger JL, Gemmell CG, Kalbfleisch JH (July 1990). "Pathogenicity of Staphylococcus lugdunensis, Staphylococcus schleiferi, and three other coagulase-negative staphylococci in a mouse model and possible virulence factors". Canadian Journal of Microbiology. 36 (7): 455–63. doi:10.1139/m90-080. PMID 2224644.
- 1 2 3 Yarbrough ML, Hamad Y, Burnham CA, George IA (November 2017). "Closing the Brief Case: Bacteremia and Vertebral Osteomyelitis Due to Staphylococcus schleiferi". Journal of Clinical Microbiology. 55 (11): 3309–3310. doi:10.1128/jcm.00512-17. PMC 5654921. PMID 29066569.
- 1 2 "Staphylococcal Infections - Infectious Diseases". Merck Manuals Professional Edition. Retrieved 2020-10-18.
- ↑ Foster G, Barley J (October 2007). "Staphylococcus schleiferi subspecies coagulans in dogs". The Veterinary Record. 161 (14): 496. doi:10.1136/vr.161.14.496. PMID 17921447. S2CID 39730386.
- 1 2 Bes M, Guérin-Faublée V, Freney J, Etienne J (April 2002). "Isolation of Staphylococcus schleiferi subspecies coagulans from two cases of canine pyoderma". The Veterinary Record. 150 (15): 487–8. doi:10.1136/vr.150.15.487. PMID 11995683. S2CID 45520855.
- ↑ Lee GY, Yang SJ (January 2020). "Staphylococcus schleiferi strain from canine otitis externa in Korea". Journal of Veterinary Science. 21 (1): e11. doi:10.4142/jvs.2020.21.e11. PMC 7000899. PMID 31940690.
- 1 2 3 Freney J, Brun Y, Bes M, Meugnier H, Grimont F, Grimont PA, Nervi C, Fleurette J (1988-04-01). "Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., Two Species from Human Clinical Specimens". International Journal of Systematic Bacteriology. 38 (2): 168–172. doi:10.1099/00207713-38-2-168. ISSN 0020-7713.
- ↑ Jousson O, Di Bello D, Vanni M, Cardini G, Soldani G, Pretti C, Intorre L (July 2007). "Genotypic versus phenotypic identification of staphylococcal species of canine origin with special reference to Staphylococcus schleiferi subsp. coagulans". Veterinary Microbiology. 123 (1–3): 238–44. doi:10.1016/j.vetmic.2007.02.020. PMID 17400408.
- 1 2 Calvo J, Hernández JL, Fariñas MC, García-Palomo D, Agüero J (October 2000). "Osteomyelitis caused by Staphylococcus schleiferi and evidence of misidentification of this Staphylococcus species by an automated bacterial identification system". Journal of Clinical Microbiology. 38 (10): 3887–9. doi:10.1128/jcm.38.10.3887-3889.2000. PMC 87502. PMID 11015429.
- 1 2 Kluytmans J, Berg H, Steegh P, Vandenesch F, Etienne J, van Belkum A (August 1998). "Outbreak of Staphylococcus schleiferi wound infections: strain characterization by randomly amplified polymorphic DNA analysis, PCR ribotyping, conventional ribotyping, and pulsed-field gel electrophoresis". Journal of Clinical Microbiology. 36 (8): 2214–9. doi:10.1128/jcm.36.8.2214-2219.1998. PMC 105016. PMID 9665994.
- ↑ Watanakunakorn C, Bakie C (May 1973). "Coagulase production, mannitol fermentation, penicillinase elaboration, and phage typability of Staphylococcus aureus reverted from L-phase variants". The Journal of Infectious Diseases. 127 (5): 571–5. doi:10.1093/infdis/127.5.571. PMID 4266897.
- ↑ Compton ST, Kania SA, Robertson AE, Lawhon SD, Jenkins SG, Westblade LF, Bemis DA (March 2017). "Evaluation of Pyrrolidonyl Arylamidase Activity in Staphylococcus delphini". Journal of Clinical Microbiology. 55 (3): 859–864. doi:10.1128/jcm.02076-16. PMC 5328453. PMID 28003425.
- ↑ Swe T, Naing AT, Baqui A, Khillan R (September 26, 2016). "coagulans Infection in a Patient With Hepatocellular Carcinoma". Journal of Investigative Medicine High Impact Case Reports. 4 (3): 2324709616671148. doi:10.1177/2324709616671148. PMC 5040195. PMID 27734018.
- ↑ Zdovc I, Ocepek M, Pirs T, Krt B, Pinter L (December 2004). "Microbiological features of Staphylococcus schleiferi subsp. coagulans, isolated from dogs and possible misidentification with other canine coagulase-positive staphylococci". Journal of Veterinary Medicine. B, Infectious Diseases and Veterinary Public Health. 51 (10): 449–54. doi:10.1111/j.1439-0450.2004.00792.x. PMID 15606869.
- 1 2 Hébert GA (November 1990). "Hemolysins and other characteristics that help differentiate and biotype Staphylococcus lugdunensis and Staphylococcus schleiferi". Journal of Clinical Microbiology. 28 (11): 2425–31. doi:10.1128/jcm.28.11.2425-2431.1990. PMC 268200. PMID 2254418.
- ↑ Misic AM, Cain CL, Morris DO, Rankin SC, Beiting DP (September 2015). "Complete Genome Sequence and Methylome of Staphylococcus schleiferi, an Important Cause of Skin and Ear Infections in Veterinary Medicine". Genome Announcements. 3 (5). doi:10.1128/genomeA.01011-15. PMC 4566178. PMID 26358596.
- 1 2 3 4 Hernández JL, Calvo J, Sota R, Agüero J, García-Palomo JD, Fariñas MC (March 2001). "Clinical and microbiological characteristics of 28 patients with Staphylococcus schleiferi infection". European Journal of Clinical Microbiology & Infectious Diseases. 20 (3): 153–8. doi:10.1007/s100960100467. PMID 11347663.
- 1 2 3 한대웅 (2018). Molecular characterization of methicillin-resistant Staphylococcus schleiferi isolated from canine skin in Korea (Thesis thesis). 서울대학교 대학원.
- ↑ Kawakami T, Shibata S, Murayama N, Nagata M, Nishifuji K, Iwasaki T, Fukata T (December 2010). "Antimicrobial susceptibility and methicillin resistance in Staphylococcus pseudintermedius and Staphylococcus schleiferi subsp. coagulans isolated from dogs with pyoderma in Japan". The Journal of Veterinary Medical Science. 72 (12): 1615–9. doi:10.1292/jvms.10-0172. PMID 20703027.
- 1 2 3 4 Vanni M, Tognetti R, Pretti C, Crema F, Soldani G, Meucci V, Intorre L (October 2009). "Antimicrobial susceptibility of Staphylococcus intermedius and Staphylococcus schleiferi isolated from dogs". Research in Veterinary Science. 87 (2): 192–5. doi:10.1016/j.rvsc.2009.01.011. PMID 19268332.
- ↑ Hariharan H, Gibson K, Peterson R, Frankie M, Matthew V, Daniels J, et al. (2014-01-23). "Staphylococcus pseudintermedius and Staphylococcus schleiferi Subspecies coagulans from Canine Pyoderma Cases in Grenada, West Indies, and Their Susceptibility to Beta-Lactam Drugs". Veterinary Medicine International. 2014: 850126. doi:10.1155/2014/850126. PMC 3922004. PMID 24592351.
- ↑ Foster G, Robb A, Paterson GK (2020). "coagulans from Antarctic and North Sea seals". Access Microbiology. 2 (10): acmi000162. doi:10.1099/acmi.0.000162. PMC 7660238. PMID 33195976.
- ↑ Eckholm NG, Outerbridge CA, White SD, Sykes JE (February 2013). "Prevalence of and risk factors for isolation of meticillin-resistant Staphylococcus spp. from dogs with pyoderma in northern California, USA". Veterinary Dermatology. 24 (1): 154–61.e34. doi:10.1111/j.1365-3164.2012.01051.x. PMID 23331692.
- ↑ Beck KM, Waisglass SE, Dick HL, Weese JS (August 2012). "Prevalence of meticillin-resistant Staphylococcus pseudintermedius (MRSP) from skin and carriage sites of dogs after treatment of their meticillin-resistant or meticillin-sensitive staphylococcal pyoderma". Veterinary Dermatology. 23 (4): 369–75, e66-7. doi:10.1111/j.1365-3164.2012.01035.x. PMID 22364707.
- ↑ Jones RD, Kania SA, Rohrbach BW, Frank LA, Bemis DA (January 2007). "Prevalence of oxacillin- and multidrug-resistant staphylococci in clinical samples from dogs: 1,772 samples (2001-2005)". Journal of the American Veterinary Medical Association. 230 (2): 221–7. doi:10.2460/javma.230.2.221. PMID 17223755.
- 1 2 3 Intorre L, Vanni M, Di Bello D, Pretti C, Meucci V, Tognetti R, et al. (October 2007). "Antimicrobial susceptibility and mechanism of resistance to fluoroquinolones in Staphylococcus intermedius and Staphylococcus schleiferi". Journal of Veterinary Pharmacology and Therapeutics. 30 (5): 464–9. doi:10.1111/j.1365-2885.2007.00896.x. PMID 17803740.
- ↑ Simou C, Hill PB, Forsythe PJ, Thoday KL (June 2005). "Species specificity in the adherence of staphylococci to canine and human corneocytes: a preliminary study". Veterinary Dermatology. 16 (3): 156–61. doi:10.1111/j.1365-3164.2005.00452.x. PMID 15960628.
- 1 2 3 Guardabassi L, Loeber ME, Jacobson A (January 2004). "Transmission of multiple antimicrobial-resistant Staphylococcus intermedius between dogs affected by deep pyoderma and their owners". Veterinary Microbiology. 98 (1): 23–7. doi:10.1016/j.vetmic.2003.09.021. PMID 14738778.
- ↑ May ER, Hnilica KA, Frank LA, Jones RD, Bemis DA (September 2005). "Isolation of Staphylococcus schleiferi from healthy dogs and dogs with otitis, pyoderma, or both". Journal of the American Veterinary Medical Association. 227 (6): 928–31. doi:10.2460/javma.2005.227.928. PMID 16190591.
- ↑ Frank LA, Kania SA, Hnilica KA, Wilkes RP, Bemis DA (February 2003). "Isolation of Staphylococcus schleiferi from dogs with pyoderma". Journal of the American Veterinary Medical Association. 222 (4): 451–4. doi:10.2460/javma.2003.222.451. PMID 12597417.
- ↑ von Eiff C, Peters G, Heilmann C (November 2002). "Pathogenesis of infections due to coagulase-negative staphylococci". The Lancet. Infectious Diseases. 2 (11): 677–85. doi:10.1016/s1473-3099(02)00438-3. PMID 12409048.
- ↑ Diederen BM, Kluytmans JA (March 2006). "The emergence of infections with community-associated methicillin resistant Staphylococcus aureus". The Journal of Infection. 52 (3): 157–68. doi:10.1016/j.jinf.2005.09.001. PMID 16289303.
- ↑ Weese JS (2005-05-01). "Methicillin-resistant Staphylococcus aureus: an emerging pathogen in small animals". Journal of the American Animal Hospital Association. 41 (3): 150–7. doi:10.5326/0410150. PMID 15870248.
- ↑ Kempker R, Mangalat D, Kongphet-Tran T, Eaton M (November 2009). "Beware of the pet dog: a case of Staphylococcus intermedius infection". The American Journal of the Medical Sciences. 338 (5): 425–7. doi:10.1097/maj.0b013e3181b0baa9. PMID 19826243.
- ↑ Tanner MA, Everett CL, Youvan DC (April 2000). "Molecular phylogenetic evidence for noninvasive zoonotic transmission of Staphylococcus intermedius from a canine pet to a human". Journal of Clinical Microbiology. 38 (4): 1628–31. doi:10.1128/JCM.38.4.1628-1631.2000. PMC 86505. PMID 10747154.
- ↑ Bannoehr J, Guardabassi L (August 2012). "Staphylococcus pseudintermedius in the dog: taxonomy, diagnostics, ecology, epidemiology and pathogenicity". Veterinary Dermatology. 23 (4): 253–66, e51-2. doi:10.1111/j.1365-3164.2012.01046.x. PMID 22515504.
- ↑ Somayaji R, Priyantha MA, Rubin JE, Church D (August 2016). "Human infections due to Staphylococcus pseudintermedius, an emerging zoonosis of canine origin: report of 24 cases". Diagnostic Microbiology and Infectious Disease. 85 (4): 471–6. doi:10.1016/j.diagmicrobio.2016.05.008. PMID 27241371.
- ↑ Blondeau LD, Rubin JE, Deneer H, Kanthan R, Sanche S, Beshard N, et al. (September 2020). "Staphylococcus pseudintermedius in a 4 month old pediatric oncology patient". Journal of Chemotherapy. 32 (5): 260–262. doi:10.1080/1120009x.2020.1773627. PMID 32538712. S2CID 219705403.
- ↑ Dye ES, Kapral FA (April 1981). "Characterization of a bactericidal lipid developing within staphylococcal abscesses". Infection and Immunity. 32 (1): 98–104. doi:10.1128/iai.32.1.98-104.1981. PMC 350593. PMID 7216498.
- ↑ Socohou A, Sina H, Degbey C, Nanoukon C, Chabi-Sika K, Ahouandjinou H, et al. (2020-08-27). "Staphylococcus spp. Strains Isolated from Surfaces and Medicotechnical Materials". International Journal of Microbiology. 2020: 6512106. doi:10.1155/2020/6512106. PMC 7474373. PMID 32908525.
- ↑ Vaudaux P, Pittet D, Haeberli A, Huggler E, Nydegger UE, Lew DP, Waldvogel FA (November 1989). "Host factors selectively increase staphylococcal adherence on inserted catheters: a role for fibronectin and fibrinogen or fibrin" (PDF). The Journal of Infectious Diseases. 160 (5): 865–75. doi:10.1093/infdis/160.5.865. PMID 2809259.
- ↑ Beceiro A, Tomás M, Bou G (April 2013). "Antimicrobial resistance and virulence: a successful or deleterious association in the bacterial world?". Clinical Microbiology Reviews. 26 (2): 185–230. doi:10.1128/CMR.00059-12. PMC 3623377. PMID 23554414.
- ↑ Klibi A, Maaroufi A, Torres C, Jouini A (December 2018). "Detection and characterization of methicillin-resistant and susceptible coagulase-negative staphylococci in milk from cows with clinical mastitis in Tunisia" (PDF). International Journal of Antimicrobial Agents. 52 (6): 930–935. doi:10.1016/j.ijantimicag.2018.07.026. PMID 30077662. S2CID 51921070.
- ↑ Hanssen AM, Ericson Sollid JU (February 2006). "SCCmec in staphylococci: genes on the move". FEMS Immunology and Medical Microbiology. 46 (1): 8–20. doi:10.1111/j.1574-695x.2005.00009.x. PMID 16420592.
- ↑ Fishovitz J, Hermoso JA, Chang M, Mobashery S (August 2014). "Penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus". IUBMB Life. 66 (8): 572–7. doi:10.1002/iub.1289. PMC 4236225. PMID 25044998.
- 1 2 Pinchuk IV, Beswick EJ, Reyes VE (August 2010). "Staphylococcal enterotoxins". Toxins. 2 (8): 2177–97. doi:10.3390/toxins2082177. PMC 3153290. PMID 22069679.
- ↑ de Oliveira Calsolari RA, Pereira VC, Araújo Júnior JP, de Souza da Cunha MD (June 2011). "Determination of toxigenic capacity by reverse transcription polymerase chain reaction in coagulase-negative staphylococci and Staphylococcus aureus isolated from newborns in Brazil". Microbiology and Immunology. 55 (6): 394–407. doi:10.1111/j.1348-0421.2011.00336.x. PMID 21434989. S2CID 205232977.
- ↑ Ortega E, Abriouel H, Lucas R, Gálvez A (August 2010). "Multiple roles of Staphylococcus aureus enterotoxins: pathogenicity, superantigenic activity, and correlation to antibiotic resistance". Toxins. 2 (8): 2117–31. doi:10.3390/toxins2082117. PMC 3153285. PMID 22069676.
- ↑ Linehan D, Etienne J, Sheehan D (May 2003). "Relationship between haemolytic and sphingomyelinase activities in a partially purified beta-like toxin from Staphylococcus schleiferi". FEMS Immunology and Medical Microbiology. 36 (1–2): 95–102. doi:10.1016/S0928-8244(03)00089-0. PMID 12727372.
- ↑ Abraham, Jill L., Daniel O. Morris, Gregory C. Griffeth, Frances S. Shofer, and Shelley C. Rankin. "Surveillance of Healthy Cats and Cats with Inflammatory Skin Disease for Colonization of the Skin by Methicillin‐resistant Coagulase‐positive Staphylococci and Staphylococcus Schleiferi Ssp. Schleiferi." Veterinary Dermatology 18.4 (2007): 252-59. Web.
- 1 2 Ihrke PJ (1996). Bacterial skin disease in the dog : a guide to canine pyoderma. Bayer AG, Business Group Animal Health. ISBN 1-884254-30-6. OCLC 35819542.
- ↑ Frank LA, Williamson NL, Wilkes RP, Kania SA, Hnilica KA, Bemis DA (August 2002). "The association of Staphylococcus schleiferi with canine pyoderma". Veterinary Dermatology. 13 (4): 211–29. doi:10.1046/j.1365-3164.2002.00298_12.x.
- ↑ "Pyoderma in Dogs - Dog Owners". Merck Veterinary Manual. Retrieved 2020-10-04.
- ↑ Cain CL, Morris DO, Rankin SC (December 2011). "Clinical characterization of Staphylococcus schleiferi infections and identification of risk factors for acquisition of oxacillin-resistant strains in dogs: 225 cases (2003-2009)". Journal of the American Veterinary Medical Association. 239 (12): 1566–73. doi:10.2460/javma.239.12.1566. PMID 22129120.
- ↑ Moriello KA. "Ear Infections and Otitis Externa in Dogs - Dog Owners". Merck Veterinary Manual. Retrieved 2020-10-04.
- 1 2 3 Pye, Charlie. "Otitis Externa in Dogs." The Canadian Veterinary Journal 59.11 (2018): 1231-1234. Web.
- ↑ Święcicka N, Bernacka H, Fac E, Zawiślak J (2015). "Prevalence and commonest causes for otitis externa in dogs from two polish veterinary clinics". Bulgarian Journal of Veterinary Medicine. 18 (1): 65–73. doi:10.15547/bjvm.824. ISSN 1311-1477.
- 1 2 Gotthelf LN (March 2004). "Diagnosis and treatment of otitis media in dogs and cats". The Veterinary Clinics of North America. Small Animal Practice. 34 (2): 469–87. doi:10.1016/j.cvsm.2003.10.007. PMID 15062620.
- ↑ "Overview of Otitis Media and Interna - Ear Disorders". Merck Veterinary Manual. Retrieved 2020-10-07.
- ↑ Jindal A, Shivpuri D, Sood S (March 2015). "Staphylococcus schleiferi meningitis in a child". The Pediatric Infectious Disease Journal. 34 (3): 329. doi:10.1097/inf.0000000000000561. PMID 25742083. S2CID 43402199.
- ↑ Jin D, Zhang S, Li M (February 2017). "Staphylococcus schleiferi Meningitis in an Infant". The Pediatric Infectious Disease Journal. 36 (2): 243–244. doi:10.1097/inf.0000000000001410. PMID 28079841. S2CID 19409654.
- ↑ Leung MJ, Nuttall N, Mazur M, Taddei TL, McComish M, Pearman JW (October 1999). "Case of Staphylococcus schleiferi endocarditis and a simple scheme to identify clumping factor-positive staphylococci". Journal of Clinical Microbiology. 37 (10): 3353–6. doi:10.1128/jcm.37.10.3353-3356.1999. PMC 85564. PMID 10488205.
- ↑ Thibodeau E, Boucher H, Denofrio D, Pham DT, Snydman D (September 2012). "First report of a left ventricular assist device infection caused by Staphylococcus schleiferi subspecies coagulans: a coagulase-positive organism". Diagnostic Microbiology and Infectious Disease. 74 (1): 68–9. doi:10.1016/j.diagmicrobio.2012.05.027. PMC 3968070. PMID 22749241.
- 1 2 Hanselman BA, Kruth SA, Rousseau J, Weese JS (September 2009). "Coagulase positive staphylococcal colonization of humans and their household pets". The Canadian Veterinary Journal. 50 (9): 954–8. PMC 2726022. PMID 19949556.
- 1 2 3 Kunder DA, Cain CL, O'Shea K, Cole SD, Rankin SC (December 2015). "Genotypic relatedness and antimicrobial resistance of Staphylococcus schleiferi in clinical samples from dogs in different geographic regions of the United States". Veterinary Dermatology. 26 (6): 406–10, e94. doi:10.1111/vde.12254. PMID 26369311.
- ↑ Sasaki T, Tsubakishita S, Kuwahara-Arai K, Matsuo M, Lu YJ, Tanaka Y, Hiramatsu K (October 2015). "Complete Genome Sequence of Methicillin-Resistant Staphylococcus schleiferi Strain TSCC54 of Canine Origin". Genome Announcements. 3 (5). doi:10.1128/genomea.01268-15. PMC 4626612. PMID 26514766.
- 1 2 3 Bierowiec K, Korzeniowska-Kowal A, Wzorek A, Rypuła K, Gamian A (2019-01-20). "Prevalence of Staphylococcus Species Colonization in Healthy and Sick Cats". BioMed Research International. 2019: 4360525. doi:10.1155/2019/4360525. PMC 6360576. PMID 30800668.
- ↑ Nelson DL (2013). Lehninger principles of biochemistry. Cox, Michael M.,, Lehninger, Albert L. (6th ed.). New York: W.H. Freeman and Company. ISBN 978-1-4292-3414-6. OCLC 824794893.
- ↑ Singhal N, Kumar M, Kanaujia PK, Virdi JS (2015-08-05). "MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis". Frontiers in Microbiology. 6: 791. doi:10.3389/fmicb.2015.00791. PMC 4525378. PMID 26300860.
- ↑ Assumpção YM, Teixeira IM, Paletta AC, Ferreira EO, Pinto TC, Penna BA (January 2020). "Matrix-assisted laser desorption ionization-time of flight mass spectrometry-based method for accurate discrimination of Staphylococcus schleiferi subspecies". Veterinary Microbiology. 240: 108472. doi:10.1016/j.vetmic.2019.108472. PMID 31902510. S2CID 208568601.
- ↑ Dubois D, Leyssene D, Chacornac JP, Kostrzewa M, Schmit PO, Talon R, et al. (March 2010). "Identification of a variety of Staphylococcus species by matrix-assisted laser desorption ionization-time of flight mass spectrometry". Journal of Clinical Microbiology. 48 (3): 941–5. doi:10.1128/JCM.00413-09. PMC 2832446. PMID 20032251.