 | |
| Names | |
|---|---|
| IUPAC name
Sodium monothiophosphate | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ECHA InfoCard | 100.030.224 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
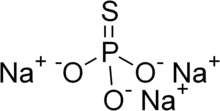
| Na3PO3S | |
| Molar mass | 180.00 g·mol−1 |
| Appearance | White solid |
| Density | 1.58 g/cm3 (dodecahydrate) 2.40 g/cm3 (anhydrous) |
| Melting point | 120 to 125 °C (248 to 257 °F; 393 to 398 K) (decomposition) |
| soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Sodium monothiophosphate, or sodium phosphorothioate, is an inorganic compound with the chemical formula Na3PO3S. It is a sodium salt of monothiophosphoric acid (H3PO3S). Sodium monothiophosphate forms hydrates Na3PO3S·xH2O. The anhydrous form and all hydrates are white solids. The anhydrous salt (x = 0) (Na3PO3S) decomposes without melting at 120-125 °C. More common is the dodecahydrate (Na3PO3S·12H2O). A nonahydrate is also known (Na3PO3S·9H2O).
Related salts are the sodium dithiophosphate undecahydrate Na3PO2S2·11H2O, sodium trithiophosphate undecahydrate Na3POS3·11H2O, and sodium tetrathiophosphate octahydrate Na3PS4·8H2O.[1]
Preparation
Sodium monothiophosphate is prepared by the base hydrolysis of thiophosphoryl chloride using aqueous sodium hydroxide:[2][3]
- PSCl3 + 6 NaOH + 9 H2O → Na3PO3S·12H2O + 3 NaCl
This reaction affords the dodecahydrate, which is easily dehydrated.
Partial dehydration over 6.5 M H2SO4 gives the nonahydrate. Under flowing N2, the anhydrous salt is formed.[4]
Sodium monothiophosphate decomposes at neutral pH. Silicone grease catalyses the hydrolysis of the monothiophosphate ion PO3S3−, so it is recommended that it is not used in the glass joints.[5]
In the anhydrous salt, the P-S bond is 211 pm and the three equivalent P-O bonds are short at 151 pm. These disparate values suggest that the P-S bond is single.[6]
References
- ↑ Elias, D. P. (1957). "Crystallographic Data on Some Sodium Phosphorothioates". Acta Crystallographica. 10 (9): 600. doi:10.1107/S0365110X57002108.
- ↑ Stanley K. Yasuda; Jack L. Lambert (1957). "Sodium Monothiophosphate". Inorganic Syntheses. Inorganic Syntheses. Vol. 5. pp. 102–104. doi:10.1002/9780470132364.ch28. ISBN 978-0-470-13236-4.
- ↑ L. C. Washburn; R. L. Hayes (1977). "Importance of Excess Base in the Synthesis of Sodium Monothiophosphate: (Sodium Phosphorothioate)". Inorganic Syntheses. Inorganic Syntheses. Vol. 17. pp. 193–4. doi:10.1002/9780470132487.ch53. ISBN 978-0-470-13248-7.
- ↑ Palazzi, Marcel (1973). "Trisodium monothiophosphate. Radiocrystallographic study". Bulletin de la Société Chimique de France. 12: 3246–8.
- ↑ Lucian C. Pop and M. Saito (2015). "Serendipitous Reactions Involving a Silicone Grease". Coordination Chemistry Reviews. 314: 64–70. doi:10.1016/j.ccr.2015.07.005.
- ↑ Pompetzki, M.; Jansen, M. (2002). "Natriummonothiophosphat(V): Kristallstruktur und Natriumionenleitfähigkeit" [Sodium monothiophosphate(V). Crystal structure and sodium ionic conductivity]. Zeitschrift für Anorganische und Allgemeine Chemie. 628 (3): 641–646. doi:10.1002/1521-3749(200203)628:3<641::AID-ZAAC641>3.0.CO;2-8.