| Response regulator receiver domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
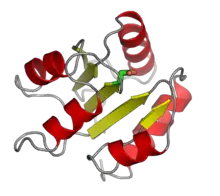 The response regulator CheY from E. coli, with the aspartate phosphorylation site highlighted in green. From PDB: 3CHY. | |||||||||
| Identifiers | |||||||||
| Symbol | Response_reg | ||||||||
| Pfam | PF00072 | ||||||||
| Pfam clan | CL0304 | ||||||||
| InterPro | IPR001789 | ||||||||
| SMART | REC | ||||||||
| PROSITE | PDOC50110 | ||||||||
| |||||||||
A response regulator is a protein that mediates a cell's response to changes in its environment as part of a two-component regulatory system. Response regulators are coupled to specific histidine kinases which serve as sensors of environmental changes. Response regulators and histidine kinases are two of the most common gene families in bacteria, where two-component signaling systems are very common; they also appear much more rarely in the genomes of some archaea, yeasts, filamentous fungi, and plants. Two-component systems are not found in metazoans.[1][2][3][4]
Function
Response regulator proteins typically consist of a receiver domain and one or more effector domains, although in some cases they possess only a receiver domain and exert their effects through protein-protein interactions. In two-component signaling, a histidine kinase responds to environmental changes by autophosphorylation on a histidine residue, following which the response regulator receiver domain catalyzes transfer of the phosphate group to its own recipient aspartate residue. This induces a conformational change that alters the function of the effector domains, usually resulting in increased transcription of target genes. The mechanisms by which this occurs are diverse and include allosteric activation of the effector domain or oligomerization of phosphorylated response regulators.[2] In a common variation on this theme, called a phosphorelay, a hybrid histidine kinase possesses its own receiver domain, and a histidine phosphotransfer protein performs the final transfer to a response regulator.[4]
In many cases, histidine kinases are bifunctional and also serve as phosphatases, catalyzing the removal of phosphate from response regulator aspartate residues, such that the signal transduced by the response regulator reflects the balance between kinase and phosphatase activity.[4] Many response regulators are also capable of autodephosphorylation, which occurs on a wide range of time scales.[2] In addition, phosphoaspartate is relatively chemically unstable and may be hydrolyzed non-enzymatically.[1]
Histidine kinases are highly specific for their cognate response regulators; there is very little cross-talk between different two-component signaling systems in the same cell.[6]
Classification
Response regulators can be divided into at least three broad classes, based on the features of effector domains: regulators with a DNA-binding effector domain, regulators with an enzymatic effector domain, and single-domain response regulators.[3] More comprehensive classifications based on more detailed analysis of domain architecture are possible. Beyond these broad categorizations, there are response regulators with other types of effector domains, including RNA-binding effector domains.
Regulators with a DNA-binding effector domain are the most common response regulators, and have direct impacts on transcription.[7] They tend to interact with their cognate regulators at an N-terminus receiver domain, and contain the DNA-binding effector towards the C-terminus. Once phosphorylated at the receiver domain, the response regulator dimerizes, gains enhanced DNA binding capacity and acts as a transcription factor.[8] The architecture of DNA binding domains are characterized as being variations on helix-turn-helix motifs. One variation, found on the response regulator OmpR of the EnvZ/OmpR two-component system and other OmpR-like response regulators, is a "winged helix" architecture.[9] OmpR-like response regulators are the largest group of response regulators and the winged helix motif is widespread. Other subtypes of DNA-binding response regulators include FixJ-like and NtrC-like regulators.[10] DNA-binding response regulators are involved in various uptake processes, including nitrate/nitrite (NarL, found in most prokaryotes).[11]
The second class of multidomain response regulators are those with enzymatic effector domains.[12] These response regulators can participate in signal transduction, and generate secondary messenger molecules. Examples include the chemotaxis regulator CheB, with a methylesterase domain that is inhibited when the response regulator is in the inactive unphosphorylated conformation. Other enzymatic response regulators include c-di-GMP phosphodiesterases (e.g. VieA in V. cholerae), protein phosphatases and histidine kinases.[12]
A relatively small number of response regulators, single-domain response regulators, only contain a receiver domain, relying on protein-protein interactions to exert their downstream biological effects.[13] The receiver domain undergoes a conformational change as it interacts with an autophosphorylated histidine kinase, and consequently, the response regulator can initiate further reactions along a signaling cascade. Prominent examples include the chemotaxis regulator CheY, which interacts with flagellar motor proteins directly in its phosphorylated state.[13]
Sequencing has so far shown that the distinct classes of response regulators are unevenly distributed throughout various taxa,[14] including across domains. While response regulators with DNA-binding domains are the most common in bacteria, single-domain response regulators are more common in archaea, with other major classes of response regulators seemingly absent from archaeal genomes.
Evolution
The number of two-component systems present in a bacterial genome is highly correlated with genome size as well as ecological niche; bacteria that occupy niches with frequent environmental fluctuations possess more histidine kinases and response regulators.[4][7] New two-component systems may arise by gene duplication or by lateral gene transfer, and the relative rates of each process vary dramatically across bacterial species.[15] In most cases, response regulator genes are located in the same operon as their cognate histidine kinase;[4] lateral gene transfers are more likely to preserve operon structure than gene duplications.[15] The small number of two-component systems present in eukaryotes most likely arose by lateral gene transfer from endosymbiotic organelles; in particular, those present in plants likely derive from chloroplasts.[4]
References
- 1 2 Stock AM, Robinson VL, Goudreau PN (2000). "Two-component signal transduction". Annual Review of Biochemistry. 69: 183–215. doi:10.1146/annurev.biochem.69.1.183. PMID 10966457.
- 1 2 3 West AH, Stock AM (June 2001). "Histidine kinases and response regulator proteins in two-component signaling systems". Trends in Biochemical Sciences. 26 (6): 369–76. doi:10.1016/s0968-0004(01)01852-7. PMID 11406410.
- 1 2 Galperin MY (June 2005). "A census of membrane-bound and intracellular signal transduction proteins in bacteria: bacterial IQ, extroverts and introverts". BMC Microbiology. 5: 35. doi:10.1186/1471-2180-5-35. PMC 1183210. PMID 15955239.
- 1 2 3 4 5 6 Capra EJ, Laub MT (2012). "Evolution of two-component signal transduction systems". Annual Review of Microbiology. 66: 325–47. doi:10.1146/annurev-micro-092611-150039. PMC 4097194. PMID 22746333.
- ↑ Zhao X, Copeland DM, Soares AS, West AH (January 2008). "Crystal structure of a complex between the phosphorelay protein YPD1 and the response regulator domain of SLN1 bound to a phosphoryl analog". Journal of Molecular Biology. 375 (4): 1141–51. doi:10.1016/j.jmb.2007.11.045. PMC 2254212. PMID 18076904.
- ↑ Rowland MA, Deeds EJ (April 2014). "Crosstalk and the evolution of specificity in two-component signaling". Proceedings of the National Academy of Sciences of the United States of America. 111 (15): 5550–5. Bibcode:2014PNAS..111.5550R. doi:10.1073/pnas.1317178111. PMC 3992699. PMID 24706803.
- 1 2 Galperin MY (June 2006). "Structural classification of bacterial response regulators: diversity of output domains and domain combinations". Journal of Bacteriology. 188 (12): 4169–82. doi:10.1128/jb.01887-05. PMC 1482966. PMID 16740923.
- ↑ Barbieri CM, Wu T, Stock AM (May 2013). "Comprehensive analysis of OmpR phosphorylation, dimerization, and DNA binding supports a canonical model for activation". Journal of Molecular Biology. 425 (10): 1612–26. doi:10.1016/j.jmb.2013.02.003. PMC 3646996. PMID 23399542.
- ↑ Kenney, Linda J (2002-04-01). "Structure/function relationships in OmpR and other winged-helix transcription factors". Current Opinion in Microbiology. 5 (2): 135–141. doi:10.1016/S1369-5274(02)00310-7. PMID 11934608.
- ↑ Rajeev L, Luning EG, Dehal PS, Price MN, Arkin AP, Mukhopadhyay A (October 2011). "Systematic mapping of two component response regulators to gene targets in a model sulfate reducing bacterium". Genome Biology. 12 (10): R99. doi:10.1186/gb-2011-12-10-r99. PMC 3333781. PMID 21992415.
- ↑ Baikalov I, Schröder I, Kaczor-Grzeskowiak M, Grzeskowiak K, Gunsalus RP, Dickerson RE (August 1996). "Structure of the Escherichia coli response regulator NarL". Biochemistry. 35 (34): 11053–61. CiteSeerX 10.1.1.580.6078. doi:10.1021/bi960919o. PMID 8780507.
- 1 2 Galperin MY (April 2010). "Diversity of structure and function of response regulator output domains". Current Opinion in Microbiology. 13 (2): 150–9. doi:10.1016/j.mib.2010.01.005. PMC 3086695. PMID 20226724.
- 1 2 Sarkar MK, Paul K, Blair D (May 2010). "Chemotaxis signaling protein CheY binds to the rotor protein FliN to control the direction of flagellar rotation in Escherichia coli". Proceedings of the National Academy of Sciences of the United States of America. 107 (20): 9370–5. Bibcode:2010PNAS..107.9370S. doi:10.1073/pnas.1000935107. PMC 2889077. PMID 20439729.
- ↑ "Census of prokaryotic response regulators". www.ncbi.nlm.nih.gov. Retrieved 2017-10-08.
- 1 2 Alm E, Huang K, Arkin A (November 2006). "The evolution of two-component systems in bacteria reveals different strategies for niche adaptation". PLOS Computational Biology. 2 (11): e143. Bibcode:2006PLSCB...2..143A. doi:10.1371/journal.pcbi.0020143. PMC 1630713. PMID 17083272.