Substituted piperazines are a class of chemical compounds based on a piperazine core.[1] Some are used as recreational drugs and some are used in scientific research.[2]
List of substituted piperazines
Benzylpiperazines
 1-Benzylpiperazine (BZP)
1-Benzylpiperazine (BZP)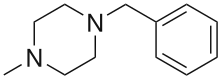 1-Methyl-4-benzylpiperazine (MBZP)
1-Methyl-4-benzylpiperazine (MBZP)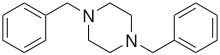 1,4-Dibenzylpiperazine (DBZP)
1,4-Dibenzylpiperazine (DBZP)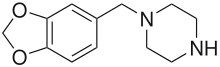
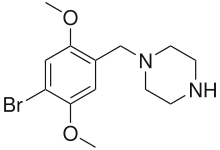 4-Bromo-2,5-dimethoxy-1-benzylpiperazine (2C-B-BZP)
4-Bromo-2,5-dimethoxy-1-benzylpiperazine (2C-B-BZP)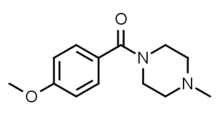 Methoxypiperamide (MeOP, MEXP) ((4-methoxyphenyl)(4-methylpiperazin-1-yl)methanone)
Methoxypiperamide (MeOP, MEXP) ((4-methoxyphenyl)(4-methylpiperazin-1-yl)methanone)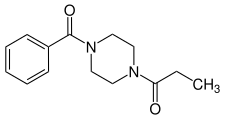 Sunifiram (1-benzoyl-4-propanoylpiperazine)
Sunifiram (1-benzoyl-4-propanoylpiperazine)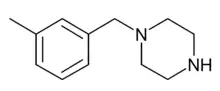 3-Methylbenzylpiperazine (3-MeBZP)
3-Methylbenzylpiperazine (3-MeBZP)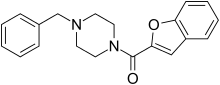 Befuraline
Befuraline
(also produces benzylpiperazine as a metabolite)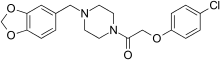 Fipexide
Fipexide
(also produces substituted benzylpiperazine as a metabolite)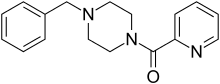 Piberaline
Piberaline
(also produces benzylpiperazine as a metabolite)
Phenylpiperazines
ortho-Substituted
- 2-Chlorophenylpiperazine (oCPP)
- 2-Methylphenylpiperazine (oMPP)
- 2-Methoxyphenylpiperazine (oMeOPP)
- Vortioxetine
Enpiprazole is known to produce oCPP as a metabolite.
Enciprazine was initially anticipated to produce oMeOPP as a metabolite, but this turned out not to be the case.
meta-Substituted
- 3-Chlorophenylpiperazine (mCPP)
- 3-Methoxyphenylpiperazine (mMeOPP)
- 3-Trifluoromethylphenylpiperazine (TFMPP)
- 1-(3-Chlorophenyl)-4-(2-phenylethyl)piperazine (3C-PEP)
Trazodone, nefazodone, mepiprazole, and others produce mCPP as a metabolite.
para-Substituted
- 4-Chlorophenylpiperazine (pCPP)
- 4-Fluorophenylpiperazine (pFPP)
- 4-Methylphenylpiperazine (pMPP)
- 4-Methoxyphenylpiperazine (MeOPP)
- 4-Nitrophenylpiperazine (pNPP)
- 4-Trifluoromethylphenylpiperazine (pTFMPP)
Multiple substitutions
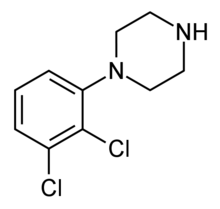 2,3-Dichlorophenylpiperazine (2,3-DCPP)
2,3-Dichlorophenylpiperazine (2,3-DCPP) 3,4-Dichlorophenylpiperazine (3,4-DCPP)
3,4-Dichlorophenylpiperazine (3,4-DCPP)
- 2,3-Methylphenylpiperazine (DMPP)
- 3-Trifluoromethyl-4-chlorophenylpiperazine (TFMCPP)
Others
- 1-Phenylpiperazine (PP)
Other arylpiperazines
- 1-(1-Naphthyl)piperazine (1-NP)
- 1-(2-Pyrimidinyl)piperazine (1-PP)
- ORG-12962 (1-(5-trifluoromethyl-6-chloropyridin-2-yl)piperazine)
- Quipazine (2-piperazin-1-ylquinoline)
Many azapirones such as buspirone, gepirone, and tandospirone produce 1-PP as a metabolite.
See also
References
- ↑ Laras, Y.; Garino, C.; Dessolin, J.; Weck, C.; Moret, V.; Rolland, A.; Kraus, J.-L. (2009-02-01). "New N4-substituted piperazine naphthamide derivatives as BACE-1 inhibitors". Journal of Enzyme Inhibition and Medicinal Chemistry. 24 (1): 181–187. doi:10.1080/14756360802048939. ISSN 1475-6366. PMID 18770069. S2CID 85385527.
- ↑ Alghamdi, Saad; Alshehri, Mohammed M.; Asif, Mohammad (2022). "The Neuropharmacological Potential of Piperazine Derivatives: A Mini- Review". Mini-Reviews in Organic Chemistry. 19 (7): 798–810. doi:10.2174/1570193x19666220119120211.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.