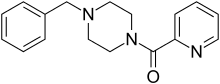
 | |
| Clinical data | |
|---|---|
| Trade names | Trelibet |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H19N3O |
| Molar mass | 281.359 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Piberaline (EGYT-475; Trelibet) is a psychoactive drug and member of the piperazine chemical class which was developed in the 1980s. It has stimulant and antidepressant effects which are thought to be due largely to its active metabolite benzylpiperazine.[1] It was researched to a limited extent in Hungary and Spain, but was not widely accepted and does not seem to be in current use, although a closely related drug befuraline with similar effects has been slightly more successful.
Synthesis
2-Chloropyridine, carbon monoxide & BZP reacted together.
Or from picolinic acid & BZP.
See also
References
- ↑ Tekes K, Tóthfalusi L, Malomvölgyi B, Hermán F, Magyar K (1987). "Studies on the biochemical mode of action of EGYT-475, a new antidepressant". Polish Journal of Pharmacology and Pharmacy. 39 (2): 203–11. PMID 2448760.
- ↑ Jenoe Dr. Koroesi, 5 More », DE 2215545 (1975 to Egyt Gyogyszervegyeszeti Gyar, Budapest).
- ↑ Kumar, Kamal; Michalik, Dirk; Garcia Castro, Ivette; Tillack, Annegret; Zapf, Alexander; Arlt, Michael; Heinrich, Timo; Böttcher, Henning; Beller, Matthias (2004). "Biologically Active Compounds through Catalysis: Efficient Synthesis ofN-(Heteroarylcarbonyl)-N′-(arylalkyl) piperazines". Chemistry - A European Journal. 10 (3): 746–757. doi:10.1002/chem.200305327.
- ↑ Younes, S (2000). "Synthesis and structure–activity relationships of novel arylalkyl 4-benzyl piperazine derivatives as σ site selective ligands". European Journal of Medicinal Chemistry. 35 (1): 107–121. doi:10.1016/S0223-5234(00)00113-6.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
