 | |
| Names | |
|---|---|
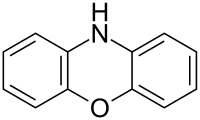
| Preferred IUPAC name
10H-Phenoxazine[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.004.737 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H9NO | |
| Molar mass | 183.210 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Phenoxazine is a heterocyclic compound. The structure of phenoxazine consists of an oxazine fused to two benzene rings. It occurs as the central core of a number of naturally occurring chemical compounds such as dactinomycin[2] and litmus. The dyes Nile blue and Nile red are also based on a phenoxazine core.
Phenoxazine dyes were once widely used for silk dyeing, but due to their lack of lightfastness they have disappeared over time from the market. However, since their light resistance is significantly better on acrylic fibers, these dyes have experienced a renaissance.
See also
References
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 216. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ "Five- and six-membered rings with two or more heteroatoms". britannica.com.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.