 | |
| Names | |
|---|---|
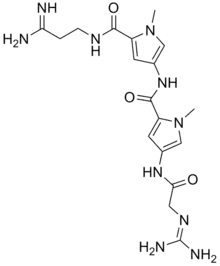
| IUPAC name
N-{5-[(3-Amino-3-iminopropyl)carbamoyl]-1-methyl-1H-pyrrol-3-yl}-4-[(N-carbamimidoylglycyl)amino]-1-methyl-1H-pyrrole-2-carboxamide | |
| Other names
Nt, congocidin, sinanomycin | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.162.288 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H26N10O3 · 2HCl | |
| Molar mass | 503.39 g/mol |
| Appearance | White powder |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Netropsin (also termed congocidine or sinanomycin[1]) is a polyamide with antibiotic and antiviral activity. Netropsin was discovered by Finlay et al., and first isolated from the actinobacterium Streptomyces netropsis.[2] It belongs to the class of pyrrole-amidine antibiotics.
DNA binding properties
Netropsin binds to the minor groove of AT-rich sequences of double stranded DNA.[3] In contrast, netropsin does not bind single stranded DNA or double stranded RNA. Crystallographic structures of DNA-bound Netropsin have been obtained and elucidate details of how the drug binds in the minor groove.[4][5] In the bound structure, the drug makes hydrogen bonding interactions with four subsequent base pairs of the DNA duplex, locally displacing the water molecules of the spine of hydration.
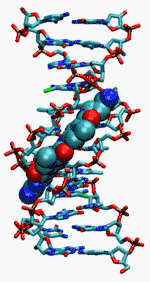
Using gel mobility and analytical ultracentrifugation, it was shown that Netropsin binding to DNA increases the twist per base by similar to 9˚ per molecule bound.[6][7] Thus, it removes supercoils when interacting with positively supercoiled DNA and introduces (additional) negative supercoils when binding to relaxed or negatively supercoiled DNA. Netropsin's effect on supercoiled DNA was observed in detail on single molecules using a magnetic tweezers.[8]
Antibiotic properties
It has been shown that Netropsin is active both against Gram-positive bacteria and Gram-negative bacteria.[9]
See also
References
- ↑ Netropsin dihydrochloride at Sigma-Aldrich
- ↑ A.C. Finlay, F. A. Hochstein, B. A. Sobin, and F. X. Murphy, J. Am. Chem Soc. 73 341-343 (1951)
- ↑ C. Zimmer and U. Wähnert, Prog. Biophys. Molec. Biol. 47 31-112 (1986)
- ↑ M. L. Kopka, C. Yoon, D. Goodsell, P. Pjura, and R.E. Dickerson, J. Mol. Biol. 183 553-563 (1985)
- ↑ M. L. Kopka, C. Yoon, D. Goodsell, P. Pjura, and R.E. Dickerson, Proc. Natl. Acad. Sci. USA 82 1376-1380 (1985)
- ↑ G. Snounou and A. D. B. Malcolm, J. Mol. Biol. 167 211-216 (1983)
- ↑ H. Triebel, H. Bär, R. Geuther, and G. Burckhardt, Progr. Colloid. Polym. Sci. 99 45-54 (1995)
- ↑ J. Lipfert, S. Klijnhout, and Nynke H. Dekker, "Nucleic Acids Res." "38" 7122-32 (2010)
- ↑ C. Zimmer and U. Wähnert, Prog. Biophys. Molec. Biol. 47 31-112 (1986)