 | |
| Names | |
|---|---|
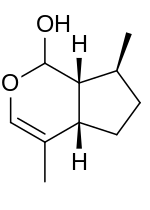
| IUPAC name
(4aS,7S,7aR)-4,7-Dimethyl-1,4a,5,6,7,7a-hexahydrocyclopenta[c]pyran-1-ol | |
| Identifiers | |
| |
3D model (JSmol) |
|
| 3DMet | |
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| |
| |
| Properties | |
| C10H16O2 | |
| Molar mass | 168.236 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Nepetalactol is an iridoid. It is produced from 8-oxogeranial by the enzyme iridoid synthase.[1] Nepetalactol is a substrate for the enzyme iridoid oxidase (IO) which produces 7-deoxyloganetic acid. It has been identified in Actinidia polygama (the silver vine) as a major cat attractant, and a mosquito repellent. The fact that mosquitos bite cats with nepetalactol on their fur less often may explain why cats are attracted to silver vine in the first place.[2]
References
- ↑ Geu-Flores, Fernando; Sherden, Nathaniel H.; Courdavault, Vincent; Burlat, Vincent; Glenn, Weslee S.; Wu, Cen; Nims, Ezekiel; Cui, Yuehua; o’Connor, Sarah E. (2012). "An alternative route to cyclic terpenes by reductive cyclization in iridoid biosynthesis". Nature. 492 (7427): 138–42. Bibcode:2012Natur.492..138G. doi:10.1038/nature11692. PMID 23172143. S2CID 4431685.
- ↑ Uenoyama, Reiko; Miyazaki, Tamako; Hurst, Jane L.; Beynon, Robert J.; Adachi, Masaatsu; Murooka, Takanobu; Onoda, Ibuki; Miyazawa, Yu; Katayama, Rieko; Yamashita, Tetsuro; Kaneko, Shuji; Nishikawa, Toshio; Miyazaki, Masao (2021). "The characteristic response of domestic cats to plant iridoids allows them to gain chemical defense against mosquitoes". Science Advances. 7 (4): eabd9135. doi:10.1126/sciadv.abd9135. PMC 7817105. PMID 33523929. S2CID 231681044.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.