 | |
| Names | |
|---|---|
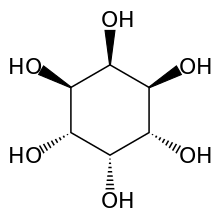
| IUPAC name
neo-Inositol[1] | |
| Systematic IUPAC name
(1R,2R,3s,4S,5S,6s)-Cyclohexane-1,2,3,4,5,6-hexol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| UNII | |
| |
| |
| Properties | |
| C6H12O6 | |
| Molar mass | 180.156 g·mol−1 |
| Melting point | 315 °C; 599 °F; 588 K[2] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Irritating to eyes, respiratory system and skin.[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
neo-Inositol is one of the stereoisomers of inositol. It is one of the nine isomeric forms of cyclohexanehexol; a group of small and chemically very stable polar molecules that have versatile properties.[4] This stereoisomer is naturally occurring, but only in small amounts. It is also known as (1s,2R,3R,4s,5S,6S)-cyclohexane-1,2,3,4,5,6-hexol or 1,2,3/4,5,6-cyclohexanehexol in the IUPAC naming system.[5]
See also
References
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 1415. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ Watt, S. W.; Chisholm, J. A.; Jones, W.; Motherwell, S. (2004). "A Molecular Dynamics Simulation of the Melting Points and Glass Transition Temperatures of Myo- and Neo-Inositol". Journal of Chemical Physics. 121 (19): 9565–9573. doi:10.1063/1.1806792. PMID 15538878.
- ↑ "Material Safety Data Sheet". Sigma-Aldrich. Retrieved 9 October 2012.
- ↑ Michell, R. H. (February 2008). "Inositol Derivatives: Evolution and Functions" (PDF). Nature Reviews Molecular Cell Biology. 9 (2): 151–61. doi:10.1038/nrm2334. PMID 18216771.
- ↑ "Neo-Inositol". Retrieved 9 October 2012.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.