| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1-Chloropyrrolidine-2,5-dione | |||
| Other names
Chlorosuccinimide | |||
| Identifiers | |||
3D model (JSmol) |
|||
| Abbreviations | NCS | ||
| 113915 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.436 | ||
| EC Number |
| ||
| 122816 | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C4H4ClNO2 | |||
| Molar mass | 133.53 g·mol−1 | ||
| Appearance | white solid | ||
| Density | 1.65 g/cm3 | ||
| Melting point | 148 to 150 °C (298 to 302 °F; 421 to 423 K) | ||
| Hazards | |||
| GHS labelling: | |||
  | |||
| Danger | |||
| H302, H314, H319, H335, H412 | |||
| P260, P261, P264, P270, P271, P273, P280, P301+P312, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P312, P321, P330, P337+P313, P363, P403+P233, P405, P501 | |||
| Related compounds | |||
Related Imides |
Succinimide N-Bromosuccinimide N-Iodosuccinimide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
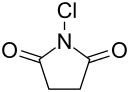
N-Chlorosuccinimide ("NCS")is the organic compound with the formula C2H4(CO)2NCl. A white solid, it is used for chlorinations.[2] It is also used as a mild oxidant.[3] NCS is related to succinimide, but with N-Cl in place of N-H. The N–Cl bond is highly reactive, and NCS functions as a source of "Cl+".
Synthesis and selected reactions
NCS is produced from succinimide by treatment with Cl+ sources, such as bleach (sodium hypochlorite), and t-butylhypochlorite, and even chlorine.[2]
Electron-rich arenes are readily monochlorinated by NCS. Aniline and mesitylene are converted to the respective chlorinated derivatives.[2]
Related reagents
- N-Iodosuccinimide (NIS), the iodine analog of N-chlorosuccinimide.[4]
- N-bromosuccinimide (NBS), the bromine analog of N-chlorosuccinimide.[5]
- Other N-chloro compounds that are commercially available include chloramine-T, trichloroisocyanuric acid ((OCNCl)3), 1,3-dichloro-5,5-dimethylhydantoin.[2]
Further reading
- Delaney, Paul A.; R. Johnstone (1985). "Solvent effects in the chlorination of tetrahydrothiophens with N-chlorosuccinimide". Tetrahedron. 41 (18): 3845–3851. doi:10.1016/S0040-4020(01)91405-X.
- N-chlorosuccinimide has been employed as chlorinating agent in combination with visible-light photoredox catalysts in organic synthesis.[6]
References
- ↑ N-Chlorosuccinimide at Sigma-Aldrich
- 1 2 3 4 Golebiewski, W. Marek; Gucma, Miro slaw (2007). "Applications of N-chlorosuccinimide in organic synthesis". Synthesis: 3599–3619. doi:10.1055/s-2007-990871.
- ↑ Kim, Kwan Soo; I. Cho; B. Yoo; Y. Song; C. Hahn (1984). "Selective oxidation of primary and secondary alcohols using di-isopropyl sulphide–N-chlorosuccinimide". J. Chem. Soc., Chem. Commun. (12): 762–763. doi:10.1039/C39840000762.
- ↑ Beebe, T. R.; R. L. Adkins; C. C. Bogardus; B. Champney; P. S. Hii; P. Reinking; J. Shadday; W. D. Weatherford; M. W. Webb; S. W. Yates (1983). "Primary alcohol oxidation with N-iodosuccinimide". J. Org. Chem. 48 (18): 3126–3128. doi:10.1021/jo00166a046.
- ↑ Castanet, Anne-Sophie; F. Colobert; P. Broutin (2002). "Mild and regioselective iodination of electron-rich aromatics with N-iodosuccinimide and catalytic trifluoroacetic acid". Tetrahedron Lett. 43 (29): 5047–5048. doi:10.1016/S0040-4039(02)01010-9.
- ↑ Rogers, David A.; Gallegos, Jillian M.; Hopkins, Megan D.; Lignieres, Austin A.; Pitzel, Amy K.; Lamar, Angus A. (2019-09-06). "Visible-light photocatalytic activation of N-chlorosuccinimide by organic dyes for the chlorination of arenes and heteroarenes". Tetrahedron. 75 (36): 130498. doi:10.1016/j.tet.2019.130498. ISSN 0040-4020.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.