 | |
| Names | |
|---|---|
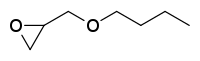
| Preferred IUPAC name
2-(Butoxymethyl)oxirane | |
| Other names
1,2-Epoxy-3-butoxypropane 2,3-Epoxypropyl butyl ether (Butoxymethyl)oxirane 1-Butoxy-2,3-epoxypropane | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.017.616 |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1993 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H14O2 | |
| Molar mass | 130.187 g·mol−1 |
| Appearance | Colorless liquid[1] |
| Odor | Irritating[1] |
| Density | 0.91 g/cm3[1] |
| Boiling point | 164 °C; 327 °F; 437 K[1] |
| 2% (20 °C)[1] | |
| Vapor pressure | 3 mmHg (25 °C)[1] |
| Hazards | |
| Flash point | 130 °F[1] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
260 mg/m3 (inhalation, mouse)[2] 1030 ppm (inhalation, rat, 8 hours)[2] |
LC50 (median concentration) |
>3500 ppm (mouse, 4 hr)[3] 1030 ppm (rat, 8 hr)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 50 ppm (270 mg/m3)[1] |
REL (Recommended) |
5.6 ppm (30 mg/m3) [15 min][1] |
IDLH (Immediate danger) |
250 ppm[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
n-Butyl glycidyl ether is an industrial chemical used in adhesives, sealants, and as a paint or coating additive. It is principally used to reduce the viscosity of epoxy resin systems.[4]
Synthesis
n-Butyl alcohol and epichlorohydrin react in a condensation reaction to form a halohydrin. This is followed by a caustic dehydrochlorination, to form n-butyl glycidyl ether.[5]
Metabolism
n-Butyl glycidyl ether is metabolized renally to butoxyacetic acid, 3-butoxy-2-hydroxypropionic acid and 3-butoxy-2-acetylaminopropionic acid.[5][6]
Safety
Exposure to n-butyl glycidyl ether through inhalation, eye contact, or skin exposure can cause a cough, sore throat, eye and skin redness, and pain. It is flammable and reacts with strong oxidants, strong bases, strong acids, and amines.[7]
Uses
As an Epoxy modifier it is classed as an epoxy Reactive diluent.[8] It is also used to synthesize other molecules.[9] The use of the diluent does effect mechanical properties and microstructure of epoxy resins.[10][11] It has been used to simultaneously increase cryogenic strength, ductility and impact resistance of epoxy resins.[12]
References
- 1 2 3 4 5 6 7 8 9 10 NIOSH Pocket Guide to Chemical Hazards. "#0081". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 4 5 6 7 8 "Propane, 1-Butoxy-2,3-epoxy". CDC/NIOSH. 28 March 2018.
- 1 2 3 "{{{2}}}". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ↑ Jagtap, Ameya Rajendra; More, Aarti (2022-08-01). "Developments in reactive diluents: a review". Polymer Bulletin. 79 (8): 5667–5708. doi:10.1007/s00289-021-03808-5. ISSN 1436-2449. S2CID 235678040.
- 1 2 CID 17049 from PubChem
- ↑ Eadsforth, C. V.; Hutson, D. H.; Logan, C. J.; Morrison, B. J. (1985). "The metabolism of n-butyl glycidyl ether in the rat and rabbit". Xenobiotica. 15 (7): 579–89. doi:10.3109/00498258509045887. PMID 4049898.
- ↑ International Chemical Safety Card 0115
- ↑ Monte, Salvatore J. (1998), Pritchard, Geoffrey (ed.), "Diluents and viscosity modifiers for epoxy resins", Plastics Additives: An A-Z reference, Polymer Science and Technology Series, Dordrecht: Springer Netherlands, vol. 1, pp. 211–216, doi:10.1007/978-94-011-5862-6_24, ISBN 978-94-011-5862-6, retrieved 2022-03-29
- ↑ Urata, Kouichi; Takaishi, Naotake (September 1994). "The alkyl glycidyl ether as synthetic building blocks". Journal of the American Oil Chemists' Society. 71 (9): 1027–1033. doi:10.1007/BF02542274. S2CID 96776835.
- ↑ Pastarnokienė, Liepa; Jonikaitė-Švėgždienė, Jūratė; Lapinskaitė, Neringa; Kulbokaitė, Rūta; Bočkuvienė, Alma; Kochanė, Tatjana; Makuška, Ričardas (2023-07-01). "The effect of reactive diluents on curing of epoxy resins and properties of the cured epoxy coatings". Journal of Coatings Technology and Research. 20 (4): 1207–1221. doi:10.1007/s11998-022-00737-4. ISSN 1935-3804. S2CID 256749849.
- ↑ Khalina, Morteza; Beheshty, Mohammad Hosain; Salimi, Ali (2019-08-01). "The effect of reactive diluent on mechanical properties and microstructure of epoxy resins". Polymer Bulletin. 76 (8): 3905–3927. doi:10.1007/s00289-018-2577-6. ISSN 1436-2449. S2CID 105389177.
- ↑ Chen, Zhen-Kun; Yang, Guo; Yang, Jiao-Ping; Fu, Shao-Yun; Ye, Lin; Huang, Yong-Gang (2009-02-23). "Simultaneously increasing cryogenic strength, ductility and impact resistance of epoxy resins modified by n-butyl glycidyl ether". Polymer. 50 (5): 1316–1323. doi:10.1016/j.polymer.2008.12.048. ISSN 0032-3861.
Further reading
- Epoxy resin technology. Paul F. Bruins, Polytechnic Institute of Brooklyn. New York: Interscience Publishers. 1968. ISBN 0-470-11390-1. OCLC 182890.
{{cite book}}: CS1 maint: others (link) - Flick, Ernest W. (1993). Epoxy resins, curing agents, compounds, and modifiers : an industrial guide. Park Ridge, NJ. ISBN 978-0-8155-1708-5. OCLC 915134542.
{{cite book}}: CS1 maint: location missing publisher (link) - Lee, Henry (1967). Handbook of epoxy resins. Kris Neville ([2nd, expanded work] ed.). New York: McGraw-Hill. ISBN 0-07-036997-6. OCLC 311631322.