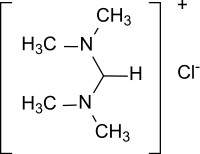
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1-(Dimethylamino)-N,N-dimethylmethaniminium chloride | |
| Other names
(Dimethylaminomethylene)dimethylammonium chloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.155.312 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H13ClN2 | |
| Molar mass | 136.62 g·mol−1 |
| Appearance | Solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
N,N,N′,N′-Tetramethylformamidinium chloride is the simplest representative of quaternary formamidinium cations of the general formula [R2N−CH=NR2]+ with a chloride as a counterion in which all hydrogen atoms of the protonated formamidine [HC(=NH2)NH2]+ are replaced by methyl groups.[1]
Deprotonation results in the exceptionally basic bis(dimethylamino)carbene R2N−C̈−NR2.[2]
Preparation
It is generated by protonation of (CH3)3COCH(N(CH3)2)2 (Bredereck's reagent).[3]
- (CH3)3COCH(N(CH3)2)2 + H+ → (CH3)3COH + [CH(N(CH3)2)2]+
N,N,N′,N′-Tetramethylformamidinium chloride is also obtained in high yield (95%) in the reaction of dimethylformamide (DMF) with dimethylcarbamoyl chloride[4]The conversion of DMF with thionyl chloride in a ratio of 3:1 obtains the product in a is significantly lower yield (72%) which appears, however, more realistic in view of the tricky handling of the chloride salt.[5]
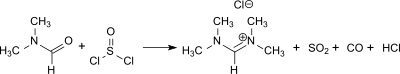 Synthesis of N,N,N′,N′-tetramethylformamidinium chloride with thionyl chloride
Synthesis of N,N,N′,N′-tetramethylformamidinium chloride with thionyl chloride
Properties
N,N,N′,N′-Tetramethylformamidinium chloride is a light yellow, strongly hygroscopic solid.[6]
For drying, the salt is dissolved in dichloromethane and the solution is treated with solid anhydrous sodium sulfate. After several dissolutions in dichloromethane and acetone, and precipitations with tetrahydrofuran, a colorless solid is obtained, which is stable under air and moisture sealing.[1]
The presumption of a mesomeric equilibrium between ionic formamidinium chloride and covalent bis(dimethylamino)chloromethane structure:
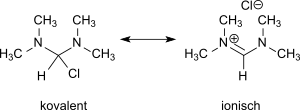 Mesomerism of N,N,N′,N′-tetramethylformamidinium chloride
Mesomerism of N,N,N′,N′-tetramethylformamidinium chloride
could be decided by reaction with germanium dichloride or tin(II) chloride in favour of the presence of N,N,N′,N′-tetramethylformamidinium chloride.[7]
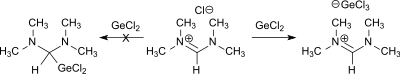 Reaction of N,N,N′,N′-tetramethylformamidinium chloride with germanium(II) chloride
Reaction of N,N,N′,N′-tetramethylformamidinium chloride with germanium(II) chloride
The hygroscopy of the chloride salt complicates the handling of the compound. Therefore, also syntheses of the much better processible salts N,N,N′,N′-tetramethylformamidinium methylsulfate[6] (from the dimethylformamide–dimethylsulfate complex[8]) and of N,N,N′,N′-tetramethylformamidinium p-toluenesulfonate (from dimethylformamide and p-toluenesulfonyl chloride) were investigated.[9][10]
Applications
N,N,N′,N′-Tetramethylformamidinium chloride is useful as a reagent for aminomethylenation (that is, to introduce a =CH−NR1R2 function to CH-acidic compounds). For example, ethyl cyanoacetate reacts with the formamidinium salt in the presence of solid sodium hydroxide to give ethyl (dimethylaminomethylene)cyanoacetate in practically quantitative yields.[11]
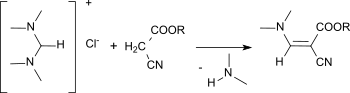 Aminomethylenation with N,N,N′,N′-tetramethylformamidinium chloride
Aminomethylenation with N,N,N′,N′-tetramethylformamidinium chloride
The aminomethylenation provides intermediates for the synthesis of heterocycles such as indoles, pyrimidines, pyridines and quinolones.
N,N,N′,N′-Tetramethylformamidinium chloride reacts with alkali metal dimethylamides (such as lithium dimethylamide or sodium dimethylamide) to tris(dimethylamino)methane in yields of 55% to 84%.[12][13][14]
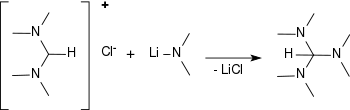 Synthesis of tris(dimethylamino)methane
Synthesis of tris(dimethylamino)methane
The reaction product is suited as a reagent for formylation and aminomethylenation.
From N,N,N′,N′-tetramethylformamidinium chloride and sodium ethoxide in ethanol, dimethylformamide diethyl acetal is formed in 68% yield.[15]
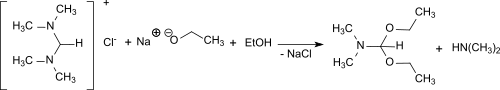 Synthesis of dimethylformamide diethyl acetal
Synthesis of dimethylformamide diethyl acetal
In aqueous sodium cyanide, N,N,N′,N′-tetramethylformamidinium reacts to bis(dimethylamino)acetonitrile.[16]
acetonitril.svg.png.webp) Synthesis of bis(dimethylamino)acetonitrile
Synthesis of bis(dimethylamino)acetonitrile
From N,N,N′,N′-tetramethylformamidinium and anhydrous hydrogen cyanide, dimethylaminomalonic acid dinitrile is obtained in 92% yield.[17]
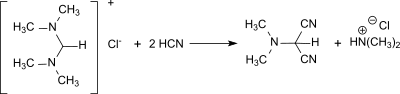 Synthesis of dimethylaminomalonic acid dinitrile
Synthesis of dimethylaminomalonic acid dinitrile
N,N,N′,N′-Tetramethylformamidinium can be regaminated with cyclo-aliphatic amines to the corresponding heterocyclic formamidines.[17]
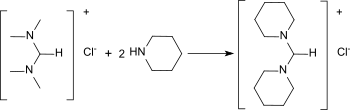 Transamination of N,N,N′,N′-tetramethylformamidinium chloride
Transamination of N,N,N′,N′-tetramethylformamidinium chloride
N,N,N′,N′-tetramethylformamidinium is a catalyst in the preparation of acyl chlorides from carboxylic acids and phosgene has been reported.[18]
Strong bases (such as phenyllithium) can abstract a proton from the formamidinium cation of N,N,N′,N′-tetramethylformamidinium forming bis(dimethylamino)carbene.[1][2]
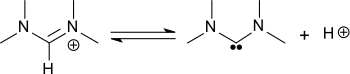 Formation of bis(dimethylamino)carbene from N,N,N′,N′-tetramethylformamidinium chloride
Formation of bis(dimethylamino)carbene from N,N,N′,N′-tetramethylformamidinium chloride
See also
References
- 1 2 3 Alder, R. W.; Blake, M. E.; Bufali, S.; Butts, C. P.; Orpen, A. G.; Schütz, J.; Williams, S. J. (2001). "Preparation of tetraalkylformamidinium salts and related species as precursors to stable carbenes". Journal of the Chemical Society, Perkin Transactions 1 (14): 1586–1593. doi:10.1039/B104110J.
- 1 2 Magill, A. M.; Cavell, K. J.; Yates, B. F. (2004). "Basicity of nucleophilic carbenes in aqueous and nonaqueous solvents – theoretical predictions". Journal of the American Chemical Society. 126 (28): 8717–24. doi:10.1021/ja038973x. PMID 15250724.
- ↑ Kantlehner, Willi; Bowers, Albert (2007). "T-Butoxybis(dimethylamino)methane". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/9780470842898.rb350.pub2. ISBN 978-0471936237.
- ↑ Arnold, Z. (1959). "The preparation of tetramethylformamidinium salts and their vinylogues". Collection of Czechoslovak Chemical Communications. 24 (3): 760–765. doi:10.1135/cccc19590760.
- ↑ Kantlehner, W.; Speh, P. (1971). "Säureamid-Reaktionen. LI. Notiz zur Darstellung von N,N,N′,N′-Tetramethylformamidiniumchlorid" [Acyl amide reactions. LI. Note on the presentation of N,N,N′,N′-tetramethylformamidinium chloride]. Chemische Berichte (in German). 104 (11): 3714–3715. doi:10.1002/cber.19711041136.
- 1 2 DE 1205528, Bredereck, H.; Effenberger, F. & Simchen, G., "Verfahren zur Herstellung von N-substituierten Amidinen oder deren Vinylogen (Process for the preparation of N-substituted amidines or their vinylogues)", issued 1965-11-25, assigned to Bredereck, H.
- ↑ Tian, X.; Pape, T.; Mitzel, N. W. (2004). "Formamidinium Salts of Low Valent Metal Halide Anions MX−
3 (M = Ge, Sn) and M
2X2−
6 (M = Ga, In)". Zeitschrift für Naturforschung. 59b (11–12): 1524–1531. doi:10.1515/znb-2004-11-1224. - ↑ Bredereck, H.; Effenberger, F.; Simchen, G. (1963). "Säureamid-Reaktionen. XXXII. Über Säureamid-Dialkylsulfat-Komplexe" [Acyl amide reactions. XXXII. On acyl amide–dialkylsulfate complexes]. Chemische Berichte (in German). 96 (5): 1350–1355. doi:10.1002/cber.19630960526.
- ↑ US 3707553, Bagley, G. E. & Poshkus A. C., "Tetramethylformamidinium arenesulfonates and method of preparation", issued 1972-12-26, assigned to Armstrong Cork Co.
- ↑ Schindlbauer, H. (1969). "Reaktionen mit Dimethylformamid. 3. (Mitt.) Die Umsetzung von Arylsulfochloriden und Arylsulfonsäuren mit Dimethylformamid" [Reactions with dimethylformamide. 3. (Comm.) The reaction of arylsulfonyl chlorides and arylsulfonic acids with dimethylformamide]. Monatshefte für Chemie (in German). 100 (5): 1590–1595. doi:10.1007/BF00900174.
- ↑ US 5241099, Blank, H.-U. & Kraus, H., "Process for the preparation of aminomethylene compounds", issued 1993-08-31, assigned to Bayer AG
- ↑ Bredereck, H.; Effenberger, F.; Brendle, T. (1966). "Synthese und Reaktionen von Trisdimethylaminomethan" [Synthesis and reactions of tris(dimethylamino)methane]. Angewandte Chemie (in German). 78 (2): 147–148. doi:10.1002/ange.19660780212.
- ↑ DE 1217391, Bredereck, H., "Verfahren zur Herstellung von Tris-dimethylaminomethan (Process for the preparation of tris(dimethylamino)methane))", issued 1966-12-08, assigned to Bredereck, H.
- ↑ Bredereck, H.; Effenberger, F.; Brendle, T.; Muffler, H. (1968). "Orthoamide. V. Synthese von Tris-dialkylamino-methanen" [Orthoamides. V. Synthesis of tris(dialkylamino)methanes]. Chemische Berichte (in German). 101 (5): 1885–1888. doi:10.1002/cber.19681010541.
- ↑ Gold, H. (1960). "Die Reaktion von Cyanurchlorid mit Dimethylformamid" [The reaction of cyanuric chloride with dimethylformamide]. Angewandte Chemie (in German). 72 (24): 956–959. doi:10.1002/ange.19600722406.
- ↑ Bredereck, H.; Simchen, G.; Kantlehner, W. (1971). "Orthoamide. XVI. Synthese von O.N- und N.N-Acetalen der α-Keto-carbonsäure-nitrile sowie von Iminoestern" [Orthoamides. XVI. Synthesis of O,N- and N,N-acetals of α-keto-carboxylic acid nitriles as well as imino esters]. Chemische Berichte (in German). 104 (3): 924–931. doi:10.1002/cber.19711040331.
- 1 2 Gold, H.; Bayer, O. (1961). "Die Darstellung basisch substituierter Malonsäure-dinitrile" [Presentation of base-substituted malonic acid dinitriles]. Chemische Berichte (in German). 94 (10): 2594–2596. doi:10.1002/cber.19610941004.
- ↑ EP 1124783, Henkelmann, J. & Stamm, A., "Method for producing carboxylic acid chlorides", issued 2001-08-22, assigned to BASF AG