 | |
| Names | |
|---|---|
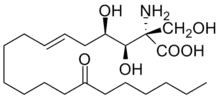
| Systematic IUPAC name
(2S,3R,4R,6E)-2-Amino-3,4-dihydroxy-2-(hydroxymethyl)-14-oxoicos-6-enoic acid | |
| Other names
Antibiotic ISP-1; Thermozymocidin | |
| Identifiers | |
3D model (JSmol) |
|
| 5113331 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.164.620 |
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2811 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H39NO6 | |
| Molar mass | 401.54 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Myriocin, also known as antibiotic ISP-1 and thermozymocidin, is a non-proteinogenic amino acid derived from certain thermophilic fungi.
Myriocin is a very potent inhibitor of serine palmitoyltransferase, the first step in sphingosine biosynthesis.[1] Due to this property, it is used in biochemical research as a tool for depleting cells of sphingolipids.
Myriocin was shown to inhibit the proliferation of an IL-2-dependent mouse cytotoxic T cell line.
Myriocin possesses immunosuppressant activity. It is reported to be 10- to 100-fold more potent than ciclosporin.
The multiple sclerosis drug fingolimod was derived from myriocin by using structure–activity relationship studies to determine the parts of the molecule important to its activity.
References
- ↑ Miyake Y, Kozutsumi Y, Nakamura S, Fujita T, Kawasaki T (1995). "Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin". Biochem. Biophys. Res. Commun. 211 (2): 396–403. doi:10.1006/bbrc.1995.1827. PMID 7794249.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.