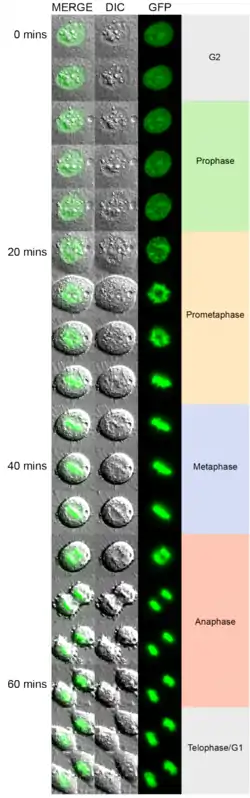
Mitotic cell rounding is a shape change that occurs in most animal cells that undergo mitosis. Cells abandon the spread or elongated shape characteristic of interphase and contract into a spherical morphology during mitosis. The phenomenon is seen both in artificial cultures in vitro and naturally forming tissue in vivo.
Early observations
In 1935, one of the first published accounts of mitotic rounding in live tissue described cell rounding in the pseudostratified epithelium of the mammalian neural tube.[1] Sauer noticed that cells in mitosis rounded up to the apical, or luminal, surface of the columnar epithelium before dividing and returning to their elongated morphology.
Significance
For a long time it was not clear why cells became round in mitosis. Recent studies in the epithelia and epidermis of various organisms, however, show that mitotic cell rounding might serve several important functions.[2]
- Firstly, mitotic cell rounding in combination with maintenance of apical cell-cell junctions appears to be necessary for correct mitotic spindle alignment, so that daughter cells divide parallel to the tissue plane, thus sharing apical surface to maintain tissue homeostasis.[3][4][5] Failure to achieve this may result in mislocalization of one daughter cell to the basal region on the tissue layer and clearance via apoptotic cell death.[5]
- Secondly, mitotic rounding has been proposed to be a driver for morphological events during tissue development. Examples include epithelial invagination of the Drosophila melanogaster tracheal placode[6] and the anisotropic shape and growth of the inner ear lumen in Zebrafish.[7]
- Thirdly, mitotic rounding has been shown to be important to generate sufficient space and appropriate geometry for proper mitotic spindle function, which is necessary for timely and accurate progression through mitosis.[2][8][9]
Thus, mitotic cell rounding is involved in tissue organization and homeostasis.
Mechanisms
To understand the physical mechanisms of how cells round up in mitosis, researchers have conducted mechanical measurements with cultured cells in vitro. The forces that drive cell rounding have recently been characterized by researchers from the groups of Professors Tony Hyman and Daniel Muller, who used flat atomic force microscopy cantilevers to constrain mitotic cells and measure the response force.[10][11] More than 90% of the forces are generated by the collective activity of myosin II molecular motors in the actin cortex.[10][11] As a result, the surface tension and effective stiffness of the actin cortex increase as has been consistently observed in mitotic cells.[12][13][14] This in turn yields an increase in intracellular hydrostatic pressure due to the Law of Laplace, which relates surface tension of a fluid interface to the differential pressure sustained across that interface.[15] The increase in hydrostatic pressure is important because it produces the outward force necessary to push and rounds up against external objects or impediments, such as flexible cantilever,[10][11] soft gel[8] or micropillar[16] (in vitro examples), or surrounding extracellular matrix and neighboring cells[7] (in vivo examples). In HeLa cells in vitro, the force generated by a half-deformed mitotic cell is on the order of 50 to 100 nanonewtons.[10][11] Internal hydrostatic pressure has been measured to increase from below 100 pascals in interphase to 3 to 10 fold that in mitosis.[10][11][15]
In similar in vitro experiments, it was found that the threshold forces required to prevent mitosis are in excess of 100 nN.[9] At threshold forces the cell suffers a loss of cortical F-actin uniformity, which further amplifies the susceptibility to applied force. These effects potentiate distortion of cell dimensions and subsequent perturbation of mitotic progression via spindle defects.[8][9]
Release of stable focal adhesions is another important aspect of mitotic rounding. Cells that are genetically perturbed to manifest constitutively active adhesion regulators are unable to properly remodel their focal adhesions and facilitate the generation of a uniform actomyosin cortex.[8][17] Overall, the biochemical events governing the morphological and mechanical changes in mitotic cells are orchestrated by the mitotic master regulator Cdk1.[11][18]
Apart from actomyosin-related genes, several disease genes have recently been implicated in mitotic cell rounding. These include Parkinson’s disease associated DJ-1/Park7 and FAM134A/RETREG2.[19]
References
- ↑ Sauer, F.C. (October 1935). "Mitosis in the neural tube". Journal of Comparative Neurology. 62 (2): 377–405. doi:10.1002/cne.900620207. S2CID 84960254.
- 1 2 Cadart, Clotilde; Zlotek-Zlotkiewicz, Ewa; Le Berre, Mael; Piel, Matthieu; Matthews, Helen K (28 April 2014). "Exploring the function of cell shape and size during mitosis". Developmental Cell. 29 (2): 159–169. doi:10.1016/j.devcel.2014.04.009. PMID 24780736.
- ↑ Meyer, Emily J; Ikmi, Aissam; Gibson, Matthew C (22 March 2011). "Interkinetic nuclear migration is a broadly conserved feature of cell division in pseudostratified epithelia". Current Biology. 21 (6): 485–491. doi:10.1016/j.cub.2011.02.002. PMID 21376598.
- ↑ Luxenburg, Chen; Pasolli, H Amalia; Williams, Scott E; Fuchs, E (20 February 2011). "Developmental roles for Srf, cortical cytoskeleton and cell shape in epidermal spindle orientation". Nature Cell Biology. 13 (3): 203–214. doi:10.1038/ncb2163. PMC 3278337. PMID 21336301.
- 1 2 Nakajima, Yu-ichiro; Meyer, Emily J; Kroesen, Amanda; McKinney, Sean A; Gibson, Matthew C (21 July 2013). "Epithelial junctions maintain tissue architecture by directing planar spindle orientation". Nature. 500 (7462): 359–362. Bibcode:2013Natur.500..359N. doi:10.1038/nature12335. PMID 23873041. S2CID 4418619.
- ↑ Kondo, Takefumi; Hayashi, Shigeo (13 January 2013). "Mitotic cell rounding accelerates epithelial invagination". Nature. 494 (7435): 125–129. Bibcode:2013Natur.494..125K. doi:10.1038/nature11792. PMID 23334416. S2CID 205232184.
- 1 2 Hoijman, Esteban; Rubbini, Davide; Colombelli, Julien; Alsina, Berta (16 June 2015). "Mitotic cell rounding and epithelial thinning regulate lumen growth and shape". Nature Communications. 6: 7355. Bibcode:2015NatCo...6.7355H. doi:10.1038/ncomms8355. hdl:10230/25942. PMID 26077034.
- 1 2 3 4 Lancaster, Oscar M; La Berre, Mael; Dimitracopoulos, Andrea; Bonazzi, Daria; Zlotek-Zlotkiewicz, Ewa; Picone, Remigio; Duke, Thomas; Piel, Matthieu; Baum, Buzz (13 May 2013). "Mitotic rounding alters cell geometry to ensure efficient bipolar spindle formation" (PDF). Developmental Cell. 25 (3): 270–283. doi:10.1016/j.devcel.2013.03.014. PMID 23623611.
- 1 2 3 Cattin, Cedric J; Düggelin, Marcel; Martinez-Martin, David; Gerber, Christoph; Mueller, Daniel J; Stewart, Martin P (2015). "Mechanical control of mitotic progression in single animal cells". Proceedings of the National Academy of Sciences. 112 (36): 11258–11263. Bibcode:2015PNAS..11211258C. doi:10.1073/pnas.1502029112. PMC 4568679. PMID 26305930.
- 1 2 3 4 5 Stewart, Martin P; Helenius, Jonne; Toyoda, Yusuke; Ramanathan, Subramanian P; Muller, Daniel J; Hyman, Anthony A (2 January 2011). "Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding". Nature. 469 (7329): 226–230. Bibcode:2011Natur.469..226S. doi:10.1038/nature09642. PMID 21196934. S2CID 4425308.
- 1 2 3 4 5 6 Ramanathan, Subramanian P; Helenius, Jonne; Stewart, Martin P; Cattin, Cedric J; Hyman, Anthony A; Muller, Daniel J (26 January 2015). "Cdk1-dependent mitotic enrichment of cortical myosin II promotes cell rounding against confinement". Nature Cell Biology. 17 (2): 148–159. doi:10.1038/ncb3098. PMID 25621953. S2CID 5208968.
- ↑ Maddox, Amy S; Burridge, Keith (20 January 2003). "RhoA is required for cortical retraction and rigidity during mitotic cell rounding". Journal of Cell Biology. 160 (2): 255–265. doi:10.1083/jcb.200207130. PMC 2172639. PMID 12538643.
- ↑ Kunda, Patricia; Pelling, Andrew E; Liu, Tao; Baum, Buzz (22 January 2008). "Moesin Controls Cortical Rigidity, Cell Rounding, and Spindle Morphogenesis during Mitosis". Current Biology. 18 (2): 91–101. doi:10.1016/j.cub.2007.12.051. PMID 18207738.
- ↑ Matthews, Helen K; Delabre, Ulysse; Rohn, Jennifer L; Guck, Jochen; Kunda, Patricia; Baum, Buzz (14 August 2012). "Changes in Ect2 localization couple actomyosin-dependent cell shape changes to mitotic progression". Developmental Cell. 23 (2): 371–383. doi:10.1016/j.devcel.2012.06.003. PMC 3763371. PMID 22898780.
- 1 2 Fischer-Friedrich, Elisabeth; Hyman, Anthony A; Jülicher, Frank; Müller, Daniel J; Helenius, Jonne (29 August 2014). "Quantification of surface tension and internal pressure generated by single mitotic cells". Scientific Reports. 4: 6213. Bibcode:2014NatSR...4E6213F. doi:10.1038/srep06213. PMC 4148660. PMID 25169063.
- ↑ Sorce, B (2015). "Mitotic cells contract actomyosin cortex and generate pressure to round against or escape epithelial confinement". Nature Communications. 6: 8872. Bibcode:2015NatCo...6.8872S. doi:10.1038/ncomms9872. hdl:1721.1/100828. PMID 26602832. S2CID 3175608.
- ↑ Dao, Vi Thuy; Dupuy, Aurélien Guy; Gavet, Olivier; Caron, Emmanuelle; de Gunzburg, Jean (15 August 2009). "Dynamic changes in Rap1 activity are required for cell retraction and spreading during mitosis". Journal of Cell Science. 122 (16): 2996–3004. doi:10.1242/jcs.041301. PMID 19638416.
- ↑ Clark, Andrew G; Paluch, Ewa (21 April 2011). "Mechanics and Regulation of Cell Shape During the Cell Cycle". Cell Cycle in Development. Results and Problems in Cell Differentiation. 53: 31–77. doi:10.1007/978-3-642-19065-0_3. ISBN 978-3-642-19064-3. PMID 21630140.
- ↑ Toyoda*, Yusuke; Cattin*, Cedric J.; Stewart*, Martin P.; Poser, Ina; Theis, Mirko; Kurzchalia, Teymuras V.; Buchholz, Frank; Hyman, Anthony A.; Müller, Daniel J. (2 November 2017). "Genome-scale single-cell mechanical phenotyping reveals disease-related genes involved in mitotic rounding". Nature Communications. 8 (1): 1266. Bibcode:2017NatCo...8.1266T. doi:10.1038/s41467-017-01147-6. PMC 5668354. PMID 29097687.
External links
- "Qucosa: The Mechanics of Mitotic Cell Rounding". qucosa.de. Archived from the original on 2015-09-24. Retrieved 2015-07-04.
- "ETH ETH E-Collection: The Mechanism of Mitotic Rounding: Role of the Actomyosin Cortex — ETH". e-collection.library.ethz.ch. Retrieved 2015-07-04.
- "Two Minute Talk: Mitotic Cell Rounding — YouTube". youtube.com. Retrieved 2015-07-04.