 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,3-Benzothiazole-2(3H)-thione | |
| Other names
Mercapto-2-benzothiazole; 2-MBT | |
| Identifiers | |
3D model (JSmol) |
|
| 508810 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.005.216 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H5NS2 | |
| Molar mass | 167.24 g·mol−1 |
| Appearance | white solid |
| Melting point | 177–181 °C (351–358 °F; 450–454 K) |
| Hazards | |
| GHS labelling: | |
  | |
| Warning | |
| H317, H410 | |
| P261, P272, P273, P280, P302+P352, P321, P333+P313, P363, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
2-Mercaptobenzothiazole is an organosulfur compound with the formula C6H4(NH)SC=S. A white solid, it is used in the sulfur vulcanization of rubber.[1]
Structure

The molecule is planar with a C=S double bond, so the name mercaptobenzothiazole is a misnomer, a more appropriate name could be benzothiazoline-2-thione. Solution measurements by NMR spectroscopy could not measure the presence of the thiol tautomer that the name implies, instead it exists as a thione/dithiocarbamate and the hydrogen appears on the nitrogen in the solid state, gas-phase, and in solution.[2] Theory indicates that the thione tautomer is about 39 kJ/mol lower in energy than the thiol, and a hydrogen-bonded dimer of the thione has even lower energy.[3] At alkaline pH greater than 7 the deprotonated thiolate form is most abundant. A protonated form could not be observed in the pH range 2-11.[4]
Synthesis
The compound has been produced by many methods. The industrial route entails the high temperature reaction of aniline and carbon disulfide in the presence of sulfur, which proceeds by this idealized equation:[3]
- C6H5NH2 + CS2 + S → C6H4(NH)SC=S + H2S
The traditional route is the reaction of 2-aminothiophenol and carbon disulfide:
- C6H4(NH2)SH + CS2 → C6H4(NH)SC=S + H2S
This method was developed by the discoverer of the compound, A. W. Hoffmann. Other routes developed by Hoffmann include the reactions of carbon disulfide with 2-aminophenol and of sodium hydrosulfide with chlorobenzothiazole.[5] Further synthetic advances were reported in the 1920s that included demonstration that phenyldithiocarbamates pyrolyze to benzothiazole derivative.[6]
Reactions
The compound is insoluble in water but dissolves upon the addition of base, reflecting deprotonation.[7] Treatment with Raney nickel results in monodesulfurization, giving benzothiazole:[3]
- C6H4(NH)SC=S + Ni → C6H4(N)SCH + NiS
The benzo ring undergoes electrophilic aromatic substitution at the position para to nitrogen.[3]
Oxidation gives mercaptobenzothiazole disulfide. This disulfide reacts with amines to give sulfenamide derivatives such 2-morpholinodithiobenzothiazole. These compounds are used in sulphur vulcanization, where they act as accelerators.
disulfide.svg.png.webp) Mercaptobenzothiazole disulfide (MBTS)
Mercaptobenzothiazole disulfide (MBTS)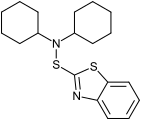 Dicyclohexyl-2-benzothiazolesulfenamide (DCBS)
Dicyclohexyl-2-benzothiazolesulfenamide (DCBS)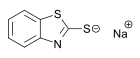 Sodium mercaptobenzothiazole
Sodium mercaptobenzothiazole
Uses
Using 2-mercaptobenzothiazole, rubber vulcanizes with less sulfur and at milder temperatures, both factors give a stronger product. This effect was reported by workers at Pirelli and at Goodyear Tire & Rubber.[1] Lorin B. Sebrell won the 1942 Charles Goodyear Medal for his work on mercaptobenzothiazole.
In polymerization, it finds use as a radical polymerization inhibitor, chain transfer agent, reforming agent, and additive for photoinitiators.[8]
The compound has also been used in the past in the gold-mining industry for the froth flotation of gold from ore residue as part of the extraction process.[9]
Sodium salt is used as a biocide and preservative in adhesives (especially based on latex, starch, casein, and animal glues), paper, textiles. Often found together with sodium dimethyldithiocarbamate as e.g. Vancide 51. Zinc salt is used as a secondary accelerator in latex foam vulcanization.[10]
It can be added to oil-based hydraulic fluids, heat-transfer fluids (oils, antifreezes), cutting fluids and other mixtures as a corrosion inhibitor, effective for copper and copper alloys.[11]
It is also used in veterinary dermatology.[12]
In electroplating it is used as a brightener for copper sulfate baths, at about 50-100 milligrams/liter. Also can be added to silver cyanide baths.[11]
Safety
Mercaptobenzothiazole has a low toxicity in mice, with LD50 of >960 mg/kg.[3]
Studies have identified it as a potential human carcinogen.[13][14] In 2016, it was identified by the World Health Organization as probably carcinogenic to humans.[15]
It causes allergic contact dermatitis.[16] The derivative morpholinylmercaptobenzothiazole is a reported allergen in protective gloves, including latex, nitrile, and neoprene gloves.[17]
It becomes air-borne as a result of wear on car tires, and is able to be inhaled.[18]
References
- 1 2 Engels, Hans-Wilhelm; Weidenhaupt, Herrmann-Josef; Pieroth, Manfred; Hofmann, Werner; Menting, Karl-Hans; Mergenhagen, Thomas; Schmoll, Ralf; Uhrlandt, Stefan (2004). "Rubber, 4. Chemicals and Additives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a23_365.pub2. ISBN 978-3527306732.
- ↑ Chesick, J. P.; Donohue, J. (1971). "The Molecular and Crystal Structure of 2-Mercaptobenzothiazole". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry. 27 (7): 1441–1444. doi:10.1107/S0567740871004102.
- 1 2 3 4 5 Wu, Feng-Ling; m. Hussein, Waleed; p. Ross, Benjamin; p. Mcgeary, Ross (2012). "2-Mercaptobenzothiazole and its Derivatives: Syntheses, Reactions and Applications". Current Organic Chemistry. 16 (13): 1555–1580. doi:10.2174/138527212800840964.
- ↑ Galvão, Tiago L. P.; Kuznetsova, Alena; Gomes, José R. B.; Zheludkevich, Mikhail L.; Tedim, João; Ferreira, Mário G. S. (March 2016). "A computational UV–Vis spectroscopic study of the chemical speciation of 2-mercaptobenzothiazole corrosion inhibitor in aqueous solution". Theoretical Chemistry Accounts. 135 (3). doi:10.1007/s00214-016-1839-3. ISSN 1432-881X. S2CID 102219996.
- ↑ A. W. Hofmann (1887). "Zur Kenntniss des o-Amidophenylmercaptans". Chem. Ber. 20: 1788–1797. doi:10.1002/cber.188702001402.
- ↑ Sebrell, L. B.; Boord, C. E. (1923). "Preparation and properties of 1-mercaptobenzothiazole, its homologs and derivatives". J. Am. Chem. Soc. 45 (10): 2390–2399. doi:10.1021/ja01663a023.
- ↑ Bashiardes, George (2005). "Benzothiazole-2-Thiol". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn00575. ISBN 0471936235.
- ↑ "2-Mercaptobenzothiazole: The Hemi-Ultra Vulcanization Accelerator".
- ↑ CABASSI, PAJ; et al. (November–December 1983). "The improved flotation of gold from the residues of Orange Free State ores" (PDF). Journal of the South African Institute of Mining and Metallurgy. 83 (11): 270–276. ISSN 0038-223X.
- ↑ Ash, Michael (2004). Handbook of Green Chemicals. Synapse Info Resources. ISBN 9781890595791.
- 1 2 "2 Mercaptobenzothiazole (MBT) Uses". 25 June 2019.
- ↑ "Mercaptobenzothiazole". drugs.com.
- ↑ T. Sorahan (April 2009). "Cancer risks in chemical production workers exposed to 2-mercaptobenzothiazole". Occup Environ Med. 66 (4): 269–273. doi:10.1136/oem.2008.041400. PMID 19158128. S2CID 3226097.
- ↑ National Toxicology Program scientists (May 1988). "NTP Toxicology and Carcinogenesis Studies of 2-Mercaptobenzothiazole (CAS No. 149-30-4) in F344/N Rats and B6C3F1 Mice (Gavage Studies)". Natl Toxicol Program Tech Rep Ser. 332: 1–172. PMID 12732904.
- ↑ Chris Graham (February 28, 2016). "Chemical found in babies' dummies and condoms 'probably causes cancer'". The Telegraph. Retrieved February 29, 2016.
- ↑ Gillian de Gannes; Sayali Tadwalkar; Aaron Wong & Nino Mebuke (2013), British Columbia Fails to Meet the North American Screening Standards: What are the Implications for Workers with Allergic Contact Dermatitis? (PDF), WorkSafeBC, archived from the original (PDF) on 2016-01-09
- ↑ Rose, R.F.; Lyons, P.; Horne, H.; Wilkinson, S.M. (2009), "A review of the materials and allergens in protective gloves", Contact Dermatitis, 61 (3): 129–137, doi:10.1111/j.1600-0536.2009.01580.x, PMID 19780770, S2CID 25877257
- ↑ Avagyan, R.; Sadiktsis, I.; Bergvall, C.; Westerholm, R. (2014), "Tire tread wear particles in ambient air—a previously unknown source of human exposure to the biocide 2-mercaptobenzothiazole", Environmental Science and Pollution Research, 21 (19): 11580–11586, doi:10.1007/s11356-014-3131-1, PMID 25028318, S2CID 9147927