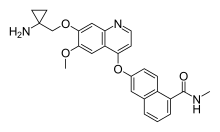
 | |
| Clinical data | |
|---|---|
| Other names | CO-3810, E-3810 |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 31–40 hrs |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C26H25N3O4 |
| Molar mass | 443.503 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Lucitanib (INN) is a drug that is being investigated by Clovis Oncology in clinical trials for the treatment of advanced solid tumours[1] including metastatic breast cancer.[2] It is a protein kinase inhibitor that blocks the VEGF receptors 1, 2 and 3, as well as the fibroblast growth factor receptors 1 and 2, and the platelet-derived growth factor receptors alpha and beta.[1]
References
- 1 2 Soria JC, DeBraud F, Bahleda R, Adamo B, Andre F, Dienstmann R, et al. (November 2014). "Phase I/IIa study evaluating the safety, efficacy, pharmacokinetics, and pharmacodynamics of lucitanib in advanced solid tumors". Annals of Oncology. 25 (11): 2244–51. doi:10.1093/annonc/mdu390. PMID 25193991.
- ↑ Clinical trial number NCT02053636 for "A Phase II Trial Testing Oral Administration of Lucitanib in Patients With Fibroblast Growth Factor Receptor (FGFR)1-amplified or Non-amplified Estrogen Receptor Positive Metastatic Breast Cancer (FINESSE)" at ClinicalTrials.gov
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.