Benzimidazole opioids, also known as nitazenes, are a class of synthetic opioids with an unusual benzimidazole structure often referred to as opioid New Psychoactive Substances (opioid NPS).[1] First synthesized in the 1950s by CIBA Pharmaceuticals as potential analgesic medications, several substances in the class have been identified, the best known being etonitazene. Like other synthetic opioids, benzimidazole opioids bind the mu-opioid receptor and may exhibit potency up to several hundred times that of morphine. While several substances in this class have found applications in research, they have never been used in clinical medicine due to their profound risk of respiratory depression and death,[2] and have in the early 2020s been recognized as emerging drugs of abuse.[3][4][5] Isotonitazine was first identified in samples of illicit drugs, and implicated in opioid overdose deaths in Europe, Canada, and the United States beginning in 2019.[6] Previously known nitazene analogs such as metonitazine and butonitazine, as well as novel nitazenes not previously patented, have since been discovered in toxicologic samples during forensic investigations.[5]
Structure-activity relationship
The structure-activity relationship of the drug class has been explored to a reasonable extent. The optimal substitution pattern is fairly tightly defined (i.e. N,N-diethyl on the amine nitrogen, 4-ethoxy on the benzyl ring and 5-nitro on the benzimidazole ring), but even derivatives incorporating only some of these features are still potent opioids. If a methyl or carboxamide group is added on the alpha carbon of the benzyl group, or the benzyl is replaced by 2-phenylethyl, compounds of similar activity are obtained. Relative analgesic activity values are derived from tests on mice and cannot be extrapolated directly to humans, though the same general activity trends apply.[7][8][9][10][11][12][13][14][15][16][17][18]
A 2019 publication[19] has shown the possibility the previously assumed binding position of the benzimidazole class,[20] acting as a semi-rigid fentanyl analogue may be incorrect. Based on a large scale analysis of known opioid receptor ligands a template was created through manual overlaying and alignment which has identified several mu-specific areas within the receptor. In this analysis, it is noted, etonitazene now more closely matches another, separate mu-specific region, sharing only a small area in common with the fentanyl class.
Abuse
In the UK, nitazene abuse emerged in 2023 as an important cause of drug-overdose death, with it being linked to 54 deaths over a 6-month period.[21] Most of the deaths have occurred outside of London, the source of supply is thought to be by post from laboratories in China, and some of the deaths have been associated by the mislabelling of nitazenes as fentanyl.
Table of benzimidazole opioids

| Chemical structure | Drug name | Ring substitution | Analgesic potency (morphine = 1) | PubChem | CAS number |
|---|---|---|---|---|---|
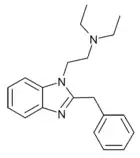 |
Desnitazene (1-diethylaminoethyl-2-benzyl-benzimidazole) | hydrogen | 0.1 | 28787 | 17817-67-3 |
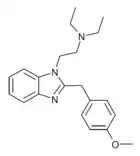 |
Metodesnitazene (Metazene) | 4-methoxy | 1 | 26412 | 14030-77-4 1071546-40-1 (HCl) |
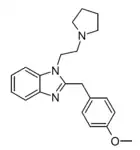 |
Metodesnitazepyne | 4-methoxy | |||
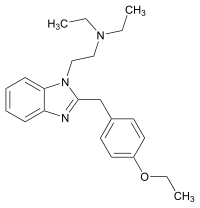 |
Etodesnitazene (Etazene) | 4-ethoxy | 70 | 149797386 | 14030-76-3 |
 |
Etodesnitazepyne | 4-ethoxy | 20 | 162623599 | |
 |
Etodesnitazepipne | 4-ethoxy | 10 | 162623611 | 102762-98-1 |
 |
Protodesnitazene | 4-(n-propoxy) | 10 | 157010653 | 805212-21-9 |
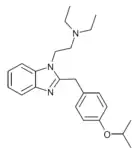 |
Isotodesnitazene | 4-isopropoxy | ~75 | 162623708 | 2732926-27-9 |
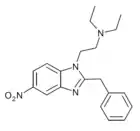 |
Nitazene | hydrogen | 2 | 15327524 | |
 |
meta-Metonitazene | 3-methoxy | 2 | ||
 |
Metonitazene | 4-methoxy | 100 | 53316366 | 14680-51-4 |
 |
Metonitazepyne | 4-methoxy | |||
 |
Metonitazepipne | 4-methoxy | |||
 |
N-Desethylmetonitazene | 4-methoxy | |||
 |
Metomethazene | 4-methoxy | |||
 |
Dimetonitazene | 3,4-dimethoxy | 10 | 162623836 | |
 |
α-methyl-metonitazene | 4-methoxy | 50 | 162625089 | 806634-80-0 |
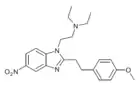 |
Metonitazene phenethyl homologue | 4-methoxy | 50 | ||
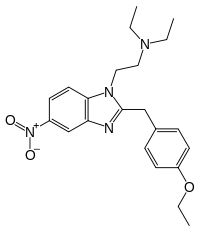 |
Etonitazene | 4-ethoxy | 1000 | 13493 | 911-65-9 |
 |
O-Desethyl-etonitazene | 4-hydroxy | 1 | 156588969 | 94758-81-3 |
 |
N-Desethyletonitazene (NDE) | 4-ethoxy | 162623580 | 2732926-26-8 | |
 |
Etonitazene 5-amino metabolite | 4-ethoxy | 2 | 13408927 | |
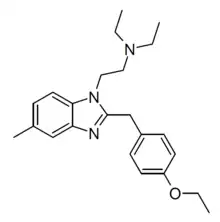 |
Etomethazene | 4-ethoxy | 20 | ||
 |
Etonitazene 5-trifluoromethyl analogue (Etotriflazene)[22] | 4-ethoxy | 21815908 | ||
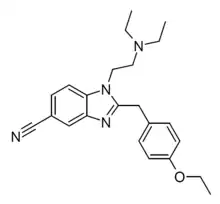 |
Etonitazene 5-cyano analogue (Etocyanazene) [23] | 4-ethoxy | 27268 | 15419-87-1 | |
 |
Etonitazene 5-acetyl analogue (Etoacetazene) [24] | 4-ethoxy | 25957 | 13406-60-5 | |
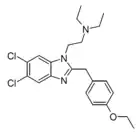 |
Etonitazene 5,6-dichloro analogue (Etodicloazene) | 4-ethoxy | |||
 |
Etonitazene N,N-dimethyl analogue | 4-ethoxy | 20 | 67089584 | 714190-52-0 |
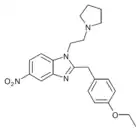 |
Etonitazepyne | 4-ethoxy | 155804760 | 2785346-75-8 | |
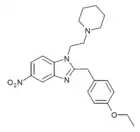 |
Etonitazepipne | 4-ethoxy | 190 [25] | 162623834 | 734496-28-7 |
 |
Etonitazene morpholine analogue | 4-ethoxy | 2 | 162623685 | 805958-08-1 |
 |
Etonitazene 6-nitro isomer (iso-etonitazene) [26] | 4-ethoxy | 20 | 59799752 | 114160-61-1 |
 |
Protonitazene | 4-(n-propoxy) | 200 | 156589001 | 119276-01-6 95958-84-2 |
 |
Protonitazepyne | 4-(n-propoxy) | 168322728 | ||
 |
Protonitazepipne | 4-(n-propoxy) | |||
 |
N-Desethylprotonitazene | 4-(n-propoxy) | |||
 |
Isotonitazene | 4-isopropoxy | 500 | 145721979 | 14188-81-9 |
 |
Isotonitazepyne | 4-isopropoxy | 168322631 | ||
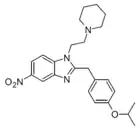 |
Isotonitazepipne | 4-isopropoxy | |||
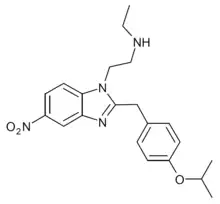 |
N-Desethylisotonitazene | 4-isopropoxy | ~1000 | 162623899 | 2732926-24-6 |
 |
Butonitazene | 4-butoxy | 5 | 156588955 | 95810-54-1 |
 |
Isobutonitazene | 4-isobutoxy | |||
 |
Secbutonitazene | 4-secbutoxy | |||
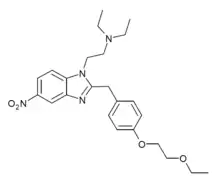 |
Etoetonitazene | 4-ethoxyethoxy | 50 | 162623504 | 806642-21-7 |
 |
Flunitazene | 4-fluoro | 1 | 156588967 | 2728-91-8 |
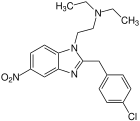 |
Clonitazene | 4-chloro | 3 | 62528 | 3861-76-5 |
 |
α-carboxamido-clonitazene | 4-chloro | 3 | ||
 |
Bronitazene | 4-bromo | 5 | 162623726 | |
 |
Methylnitazene (Menitazene) | 4-methyl | 10 | 162623683 | 95282-00-1 |
 |
Ethylnitazene (Enitazene) | 4-ethyl | 20 | 162623845 | |
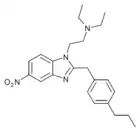 |
Propylnitazene (Pronitazene) | 4-propyl | 50 | 162623877 | 700342-00-3 |
 |
t-Butylnitazene | 4-(tert-butyl) | 2 | 162623621 | 805215-64-9 |
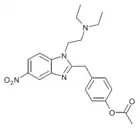 |
Acetoxynitazene | 4-acetoxy | 5 | 162623779 | 102760-24-7 |
 |
Methylthionitazene | 4-methylthio | 50 | 162623790 | 102471-37-4 |
 |
Ethylthionitazene | 4-ethylthio | 30 | 162623931 | 102758-70-3 |
 |
Etodesnitazene phenylthio analogue | 4-ethoxy | 1 | 21045 | |
 |
Etodesnitazene phenylthio / pyrrolidine analogue | 4-ethoxy | 2 | 19846499 | |
 |
Tetrahydrofuranitazene | fused tetrahydrofuran | |||
See also
References
- ↑ Ujváry I, Christie R, Evans-Brown M, Gallegos A, Jorge R, de Morais J, Sedefov R (April 2021). "DARK Classics in Chemical Neuroscience: Etonitazene and Related Benzimidazoles". ACS Chemical Neuroscience. 12 (7): 1072–1092. doi:10.1021/acschemneuro.1c00037. PMID 33760580. S2CID 232356192.
- ↑ Montanari E, Madeo G, Pichini S, Busardò FP, Carlier J (August 2022). "Acute Intoxications and Fatalities Associated With Benzimidazole Opioid (Nitazene Analog) Use: A Systematic Review". Therapeutic Drug Monitoring. 44 (4): 494–510. doi:10.1097/FTD.0000000000000970. PMID 35149665. S2CID 246776288.
- ↑ US patent 2935514, Karl Hoffman & Alfred Hunger, "BENZMDAZOLES (sic)", published 1960-05-03, assigned to Ciba Pharmaceutical Products Inc.
- ↑ Drug Enforcement Administration (June 2021). "Benzimidazole Opioids" (PDF). Retrieved 6 January 2022.
- 1 2 Walton SE, Krotulski AJ, Logan BK (March 2022). "A Forward-Thinking Approach to Addressing the New Synthetic Opioid 2-Benzylbenzimidazole Nitazene Analogs by Liquid Chromatography-Tandem Quadrupole Mass Spectrometry (LC-QQQ-MS)". Journal of Analytical Toxicology. 46 (3): 221–231. doi:10.1093/jat/bkab117. PMC 8935987. PMID 34792157.
- ↑ European Monitoring Centre for Drugs Drug Addiction (2020-11-13). "Report on the risk assessment of N,N-diethyl-2- 4-(1-methylethoxy)phenyl]methyl]-5-nitro-1Hbenzimidazole- 1-ethanamine (isotonitazene) in accordance with Article 5c of Regulation (EC) No 1920/2006 (as amended)". European Monitoring Centre for Drugs and Drug Addiction. Publications Office of the European Union. doi:10.2810/107576. ISBN 9789294974952. Retrieved 9 May 2022.
- ↑ US 2944062, Hoffman K, Hunger A, "Certain Alpha (1-diethylaminoethyl (2), Alpha Aryl Acetamides", issued 5 July 1960, assigned to Ciba Pharma Products Inc.
- ↑ Gross F, Turrian H (October 1957). "[Benzimidazole derivatives with strong analgesic effects]". Experientia. 13 (10): 401–3. doi:10.1007/BF02161117. PMID 13473818. S2CID 6824038.
- ↑ Renton P, Green B, Maddaford S, Rakhit S, Andrews JS (March 2012). "NOpiates: Novel Dual Action Neuronal Nitric Oxide Synthase Inhibitors with μ-Opioid Agonist Activity". ACS Medicinal Chemistry Letters. 3 (3): 227–31. doi:10.1021/ml200268w. PMC 4025805. PMID 24900459.
- ↑ Hunger A, Kebrle J, Rossi A, Hoffmann K (October 1957). "[Synthesis of analgesically active benzimidazole derivatives with basic substitutions]" [Synthesis of analgesically active benzimidazole derivatives with basic substitutions]. Experientia. 13 (10): 400–1. doi:10.1007/BF02161116. PMID 13473817. S2CID 32179439.
- ↑ Rossi A, Hunger A, Kebrle J, Hoffmann K (1960). "Benzimidazol-Derivate und verwandte Heterocyclen. IV. Die Kondensation von o-Phenylendiamin mit α-Aryl- und γ-Aryl-acetessigester" [Benzimidazole derivatives and related heterocycles IV. The condensation of o-phenylenediamine with α-aryl and γ-aryl-acetoacetate]. Helvetica Chimica Acta (in German). 43 (4): 1046–1056. doi:10.1002/hlca.19600430413.
- ↑ Rossi A, Hunger A, Kebrle J, Hoffmann K (1960). "Benzimidazol-Derivate und verwandte Heterocyclen V. Die Kondensation von o-Phenylendiamin mit aliphatischen und alicyclischen β-Ketoestern" [Benzimidazole derivatives and related heterocycles V. The condensation of o-phenylenediamine with aliphatic and alicyclic β-keto esters]. Helvetica Chimica Acta (in German). 43 (5): 1298–1313. doi:10.1002/hlca.19600430515.
- ↑ Hunger A, Kebrle J, Rossi A, Hoffmann K (1960). "Benzimidazol-Derivate und verwandte Heterocyclen VI. Synthese von Phenyl-[1-aminoalkyl-benzimidazolyl-(2)]-essigsäure-estern und -amiden" [Benzimidazole derivatives and related Heterocycles VI. Synthesis of phenyl-[1-aminoalkyl-benzimidazolyl-(2)]-acetic acid esters and amides]. Helvetica Chimica Acta (in German). 43 (6): 1727–1733. doi:10.1002/hlca.19600430634.
- ↑ Hunger A, Kebrle J, Rossi A, Hoffmann K (1961). "Benzimidazol-Derivate und verwandte Heterocyclen VII. Synthese neuer 2-Amino-benzimidazole" [Benzimidazole Derivatives and related Heterocycles VII. Synthesis of new 2-amino-benzimidazole]. Helvetica Chimica Acta (in German). 44 (5): 1273–1282. doi:10.1002/hlca.19610440513.
- ↑ Gross F, Turrian H (October 1957). "[Benzimidazole derivatives with strong analgesic effects]" [Benzimidazole derivatives with strong analgesic effects]. Experientia. 13 (10): 401–3. doi:10.1007/BF02161117. PMID 13473818. S2CID 6824038.
- ↑ Seki T, Sasajima M, Watanbe Y, Nakajima K (March 1967). "[Studies on 2-benzimidazolethiol derivatives. N. Analgesic effect and pharmacological property of 1-(2-diethylaminoethyl)-2-(p-ethoxyphenylthio)benzimidazole hydrochloride]". Yakugaku Zasshi (in Japanese). 87 (3): 296–301. doi:10.1248/yakushi1947.87.3_296. PMID 6069375.
- ↑ Seki T (March 1967). "[Studies on 2-benzimidazolethiol derivatives. V. Structure-activity relationship on analgesic action of 1-(dialkylamino-alkyl)-2-(p-ethoxyphenylthio)benzimidazole]". Yakugaku Zasshi (in Japanese). 87 (3): 301–9. doi:10.1248/yakushi1947.87.3_301. PMID 6069376.
- ↑ Seki T, Watanabe Y (May 1969). "[Studies on 2-benzimidazolethiol derivatives. VI. Synthesis and analgesic effect of 1-(2-diethylaminoethyl)-2-(p-ethoxyphenylthio)-5-substituted benzimidazole]". Yakugaku Zasshi (in Japanese). 89 (5): 617–26. doi:10.1248/yakushi1947.89.5_617. PMID 5817995.
- ↑ Wu Z, Hruby VJ (October 2019). "Toward a Universal μ-Agonist Template for Template-Based Alignment Modeling of Opioid Ligands". ACS Omega. 4 (17): 17457–17476. doi:10.1021/acsomega.9b02244. PMC 6812133. PMID 31656918.
- ↑ Beckett AH, Casy AF (February 1965). "Analgesics and their antagonists: biochemical aspects and structure-activity relationships". Progress in Medicinal Chemistry. 4: 171–218. doi:10.1016/s0079-6468(08)70169-3. ISBN 9780444533234. PMID 5319798.
- ↑ Homer, Alex; Johal, Navtej (11 December 2023). "Street drugs stronger than heroin linked to 54 deaths in UK". BBC News.
- ↑ Tonelli M, Cichero E, Mahmoud AM, Rabbito A, Tasso B, Fossa P, Ligresti A (December 2018). "Exploring the effectiveness of novel benzimidazoles as CB2 ligands: synthesis, biological evaluation, molecular docking studies and ADMET prediction". MedChemComm. 9 (12): 2045–2054. doi:10.1039/c8md00461g. PMC 6301267. PMID 30647880.
- ↑ Chimica Therapeutica 2(16): 1967.
- ↑ A review of the evidence on the use and harms of 2-benzyl benzimidazole (‘nitazene’) and piperidine benzimidazolone (‘brorphine-like’) opioids. Advisory Council on the Misuse of Drugs, UK. July 2022
- ↑ Vandeputte MM, Verougstraete N, Walther D, Glatfelter GC, Malfliet J, Baumann MH, Verstraete AG, Stove CP. First identification, chemical analysis and pharmacological characterization of N-piperidinyl etonitazene (etonitazepipne), a recent addition to the 2-benzylbenzimidazole opioid subclass. Arch Toxicol. 2022 Jun;96(6):1865-1880. doi:10.1007/s00204-022-03294-2 PMID 35449307
- ↑ Kanamori T, Okada Y, Segawa H, Yamamuro T, Kuwayama K, Tsujikawa K, Iwata YT (November 2022). "Analysis of highly potent synthetic opioid nitazene analogs and their positional isomers". Drug Testing and Analysis. 15 (4): 449–457. doi:10.1002/dta.3415. PMID 36437623. S2CID 254042990.