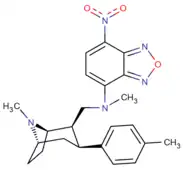
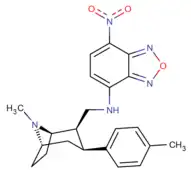
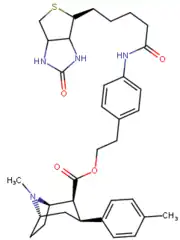
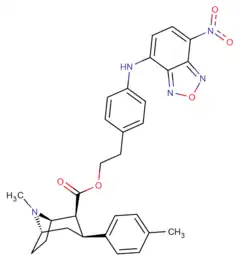
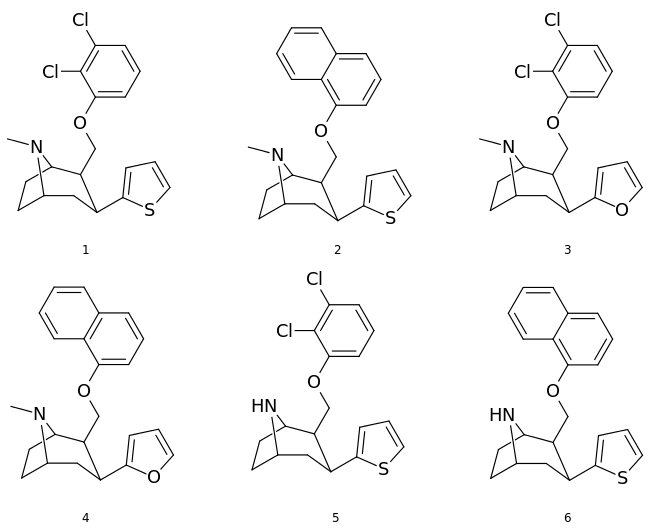
Phenyltropanes (PTs) are a family of chemical compounds originally derived from structural modification of cocaine. The main feature differentiating phenyltropanes from cocaine is that they lack the ester functionality at the 3-position terminating in the benzene; and thusly the phenyl is attached direct to the tropane skeleton with no further spacer (therefore the name "phenyl"-tropane) that the cocaine benzoyloxy provided. The original purpose of which was to extirpate the cardiotoxicity inherent in the local anesthetic "numbing" capability of cocaine (since the methylated benzoate ester is essential to cocaine's blockage of sodium channels which cause topical anesthesia) while retaining stimulant function.[lower-alpha 1] These compounds present many different avenues of research into therapeutic applications, particularly in addiction treatment. Uses vary depending on their construction and structure-activity relationship ranging from the treating of cocaine dependency to understanding the dopamine reward system in the human brain to treating Alzheimer's & Parkinson's diseases. (Since 2008 there have been continual additions to the list and enumerations of the plethora of types of chemicals that fall into the category of this substance profile.[2]) Certain phenyltropanes can even be used as a smoking cessation aid (c.f. RTI-29). Many of the compounds were first elucidated in published material by the Research Triangle Institute and are thus named with "RTI" serial-numbers (in this case the long form is either RTI-COC-n, for 'cocaine' "analog", or specifically RTI-4229-n of the subsequent numbers given below in this article)[lower-alpha 2] Similarly, a number of others are named for Sterling-Winthrop pharmaceuticals ("WIN" serial-numbers) and Wake Forest University ("WF" serial-numbers). The following includes many of the phenyltropane class of drugs that have been made and studied.


2-Carboxymethyl esters (phenyl-methylecgonines)





Like cocaine, phenyltropanes are considered a 'typical' or 'classical' (i.e. "cocaine-like") DAT re-uptake pump ligands in that they stabilize an "open-to-out" conformation on the dopamine transporter; despite the extreme similarity to phenyltropanes, benztropine and others are in suchwise not considered "cocaine-like" and are instead considered atypical inhibitors insofar as they stabilize what is considered a more inward-facing (closed-to-out) conformational state.[5]
Considering the differences between PTs and cocaine: the difference in the length of the benzoyloxy and the phenyl linkage contrasted between cocaine and phenyltropanes makes for a shorter distance between the centroid of the aromatic benzene and the bridge nitrogen of the tropane in the latter PTs. This distance being on a scale of 5.6 Å for phenyltropanes and 7.7 Å for cocaine or analogs with the benzoyloxy intact.[lower-alpha 3] The manner in which this sets phenyltropanes into the binding pocket at MAT is postulated as one possible explanation to account for PTs increased behavioral stimulation profile over cocaine.[lower-alpha 4]
Blank spacings within tables for omitted data use "no data", "?", "-" or "—" interchangeably.
Structure  |
Short Name i.e. Trivial IUPAC (non-systematic) Name (Singh's #) |
R (para-substitution) of benzene |
DA [3H]WIN 35428 IC50 nM (Ki nM) |
5HT [3H]paroxetine IC50 nM (Ki nM) |
NE [3H]nisoxetine IC50 nM (Ki nM) |
selectivity 5-HTT/DAT |
selectivity NET/DAT |
|---|---|---|---|---|---|---|---|
| cocaine (benzoyloxytropane) | H | 102 ± 12 241 ± 18ɑ | 1045 ± 89 112 ± 2b | 3298 ± 293 160 ± 15c | 10.2 0.5d | 32.3 0.7e | |
 | (para-hydrogen)phenyltropane WIN 35,065-2 (β-CPT[lower-alpha 5]) Troparil 11a | H | 23 ± 5.0 49.8 ± 2.2ɑ | 1962 ± 61 173 ± 13b | 920 ± 73 37.2 ± 5.2c | 85.3 3.5d | 40.0 0.7e |
 | para-fluorophenyltropane WIN 35,428 (β-CFT[lower-alpha 6]) 11b | F | 14 (15.7 ± 1.4) 22.9 ± 0.4ɑ | 156 (810 ± 59) 100 ± 13b | 85 (835 ± 45) 38.6 ± 9.9c | 51.6 4.4d | 53.2 1.7e |
 | para-nitrophenyltropane 11k | NO2 | 10.1 ± 0.10 | ? | ? | ? | ? |
 | para-aminophenyltropane RTI-29[6] 11j | NH2 | 9.8 24.8 ± 1.3g | 5110 | 151 | 521.4 | 15.4 |
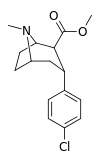
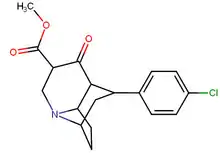
 | para-chlorophenyltropane RTI-31 11c | Cl | 1.12 ± 0.06 3.68 ± 0.09ɑ | 44.5 ± 1.3 5.00 ± 0.05b | 37 ± 2.1 5.86 ± 0.67c | 39.7 1.3d | 33.0 1.7e |
 | para-methylphenyltropane RTI-32 Tolpane 11f | Me | 1.71 ± 0.30 7.02 ± 0.30ɑ | 240 ± 27 19.38 ± 0.65b | 60 ± 0.53e 8.42 ± 1.53c | 140 2.8d | 35.1 1.2e |
 | para-bromophenyltropane RTI-51 Bromopane 11d | Br | 1.81 (1.69) ± 0.30 | 10.6 ± 0.24 | 37.4 ± 5.2 | 5.8 | 20.7 |
 | para-iodophenyltropane RTI-55 (β-CIT) Iometopane 11e | I | 1.26 ± 0.04 1.96 ± 0.09ɑ | 4.21 ± 0.3 1.74 ± 0.23b | 36 ± 2.7 7.51 ± 0.82c | 3.3 0.9d | 28.6 3.8e |
 | para-hydroxyphenyltropane 11h | OH | 12.1 ± 0.86 | — | — | — | — |
 | para-methoxyphenyltropane 11i | OCH3 | 8.14 ± 1.3 | — | — | — | — |
 | para-azidophenyltropane 11l | N3 | 2.12 ± 0.13 | — | — | — | — |
 | para-trifluoromethylphenyltropane 11m | CF3 | 13.1 ± 2.2 | — | — | — | — |
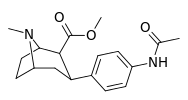 | para-acetylaminophenyltropane 11n | NHCOCH3 | 64.2 ± 2.6 | — | — | — | — |
 | para-propionylaminophenyltropane 11o | NHCOC2H5 | 121 ± 2.7 | — | — | — | — |
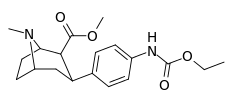 | para-ethoxycarbonylaminophenyltropane 11p | NHCO2C3H5 | 316 ± 48 | — | — | — | — |
 | para-trimethylstannylphenyltropane 11q | Sn(CH3)3 | 144 ± 37 | — | — | — | — |
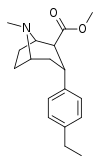
 | para-ethylphenyltropane RTI-83 11g | Et | 55 ± 2.1 | 28.4 ± 3.8 (2.58 ± 3.5) | 4030 (3910) ± 381 (2360 ± 230) | 0.5 | 73.3 |
 | para-n-propylphenyltropane RTI-282i 11r | n-C3H7 | 68.5 ± 7.1 | 70.4 ± 4.1 | 3920 ± 130 | 1.0 | 57.2 |
 | para-isopropylphenyltropane 11s | CH(CH3)2 | 597 ± 52 | 191 ± 9.5 | 75000 ± 5820 | 0.3 | 126 |
 | para-vinylphenyltropane RTI-359 11t | CH-CH2 | 1.24 ± 0.2 | 9.5 ± 0.8 | 78 ± 4.1 | 7.7 | 62.9 |
 | para-methylethenylphenyltropane RTI-283j 11u | C(=CH2)CH3 | 14.4 ± 0.3 | 3.13 ± 0.16 | 1330 ± 333 | 0.2 | 92.4 |
 | para-trans-propenylphenyltropane RTI-296i 11v | trans-CH=CHCH3 | 5.29 ± 0.53 | 11.4 ± 0.28 | 1590 ± 93 | 2.1 | 300 |
 | para-allylphenyltropane 11x | CH2CH=CH2 | 32.8 ± 3.1 | 28.4 ± 2.4 | 2480 ± 229 | 0.9 | 75.6 |
 | para-ethynylphenyltropane RTI-360 11y | C≡CH | 1.2 ± 0.1 | 4.4 ± 0.4 | 83.2 ± 2.8 | 3.7 | 69.3 |
 | para-propynylphenyltropane RTI-281i 11z | C≡CCH3 | 2.37 ± 0.2 | 15.7 ± 1.5 | 820 ± 46 | 6.6 | 346 |
 | para-cis-propenylphenyltropane RTI-304 11w | cis-CH=CHCH3 | 15 ± 1.2 | 7.1 ± 0.71 | 2,800k ± 300 | 0.5 | 186.6k |
 | para-(Z)-phenylethenylphenyltropane | cis-CH=CHPh | 11.7 ± 1.12 | — | — | — | — |
 | para-benzylphenyltropane | -CH2-Ph | 526 ± 65 | 7,240 ± 390 (658 ± 35) | 6670 ± 377 (606 ± 277) | 13.7 | 12.6 |
 | para-phenylethenylphenyltropane | CH2 ║ -C-Ph | 474 ± 133 | 2,710 ± 800 (246 ± 73) | 7,060 ± 1,760 (4,260 ± 1,060) | 5.7 | 14.8 |
 | para-phenylethylphenyltropanel | -(CH2)2-Ph | 5.14 ± 0.63 | 234 ± 26 (21.3 ± 2.4) | 10.8 ± 0.3 (6.50 ± 0.20) | 45.5 | 2.1 |
 | para-(E)-phenylethenylphenyltropanel RTI-436 | trans–CH=CHPh | 3.09 ± 0.75 | 335 ± 150 (30.5 ± 13.6) | 1960 ± 383 (1180 ± 231) | 108.4 | 634.3 |
 | para-phenylpropylphenyltropanel | -(CH2)3-Ph | 351 ± 52 | 1,243 ± 381 (113 ± 35) | 14,200 ± 1,800 (8,500 ± 1,100) | 3.5 | 40.4 |
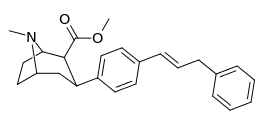 | para-phenylpropenylphenyltropanel | -CH=CH-CH2-Ph | 15.8 ± 1.31 | 781 ± 258 (71 ± 24) | 1,250 ± 100 (759 ± 60) | 49.4 | 79.1 |
 | para-phenylbutylphenyltropanel | -(CH2)4-Ph | 228 ± 21 | 4,824 ± 170 (439 ± 16) | 2,310 ± 293 (1,390 ± 177) | 21.1 | 10.1 |
 | para-phenylethynylphenyltropanel RTI-298[7] | –≡–Ph | 3.7 ± 0.16 | 46.8 ± 5.8 (4.3 ± 0.53) | 347 ± 25 (209 ± 15) | 12.6 | 93.7 |
 | para-phenylpropynylphenyltropanel[8] | –C≡C-CH2Ph | 1.82 ± 0.42 | 13.1 ± 1.7 (1.19 ± 0.42) | 27.4 ± 2.6 (16.5 ± 1.6) | 7.1 | 15 |
 | para-phenylbutynylphenyltropanel RTI-430 | –C≡C(CH2)2Ph | 6.28 ± 1.25 | 2180 ± 345 (198 ± 31) | 1470 ± 109 (885 ± 66) | 347.1 | 234 |
 | para-phenylpentynylphenyltropanel | –C≡C-(CH2)3-Ph | 300 ± 37 | 1,340 ± 232 (122 ± 21) | 4,450 ± 637 (2,680 ± 384) | 4.46 | 14.8 |
 | para-trimethylsilylethynylphenyltropane[3] | — | — | — | — | — | — |
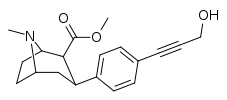 | para-hydroxypropynylphenyltropane[3] | — | — | — | — | — | — |
 | para-hydroxyhexynylphenyltropanel | –C≡C-(CH2)4OH | 57 ± 4 | 828 ± 29 (75 ± 2.6) | 9,500 ± 812 (5,720 ± 489) | 14.5 | 166.6 |
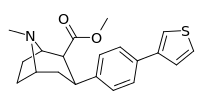 | para-(thiophen-3-yl)phenyltropane Tamagnan[4] | p-thiophene | 12 | 0.017 | 189 | 0.001416 | 15.7 |
 | para-biphenyltropane 11aa | Ph | 10.3 ± 2.6f 29.4 ± 3.8ɑ 15.6 ± 0.6 | 95.8 ± 36 (8.7 ± 3.3) | 1,480 ± 269 (892 ± 162) | 6.1 | 94.8 |
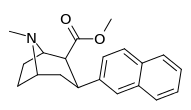 | 3β-2-naphthyltropane RTI-318 11bb | 3β-2-naphthyl | 0.51 ± 0.03 3.32 ± 0.08f 3.53 ± 0.09ɑ | 0.80 ± 0.06 (0.07 ± 0.1) | 21.1 ± 1.0 (12.7 ± 0.60) | 1.5 | 41.3 |
 | para-bimethoxyphenyltropane 15 | OCH2OCH3h | — | — | — | — | — |
|
|
|
(4′-Monosubstituted 2,3-Thiophene phenyl)-tropanes
| Compound structure | Alphanumeric code (name) |
para-substitution | N8 | SERT | DAT | NET | Selectivity SERT versus DAT |
Selectivity SERT versus NET |
|---|---|---|---|---|---|---|---|---|
| 1 (cocaine) | (—)-Cocaine | CH3 | 1050 | 89 | 3320 | 0.08 | 3.2 | |
| 2 (β-CIT), (Iometopane) | Iodo | CH3 | 0.46 ± 0.06 | 0.96 ± 0.15 | 2.80 ± 0.40 | 2.1 | 6.1 | |
| (R,S-Citalopram) | — | — | 1.60 | 16,540 | 6,190 | 10,338 | 3,869 | |
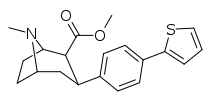 | 4a | 2-Thiophene | CH3 | 0.15 ± 0.015 | 52 ± 12.8 | 158 ± 12 | 346 | 1,053 |
 | 4b (Tamagnan) | 3-Thiophene | CH3 | 0.017 ± 0.004 | 12.1 ± 3 | 189 ± 82 | 710 | 11,118 |
 | 4c | 2-(5-Br)-Thiophene | CH3 | 0.38 ± 0.008 | 6.43 ± 0.9 | 324 ± 19 | 17 | 853 |
 | 4d | 2-(5-Cl)-Thiophene | CH3 | 0.64 ± 0.04 | 4.42 ± 1.64 | 311 ± 25 | 6.9 | 486 |
 | 4e | 2-(5-I)-Thiophene | CH3 | 4.56 ± 0.84 | 22.1 ± 3.2 | 1,137 ± 123 | 4.9 | 249 |
 | 4f | 2-(5-NH2)-Thiophene | CH3 | 64.7 ± 3.7 | >10,000 | >30,000 | >155 | >464 |
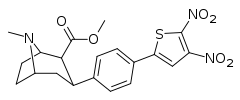 | 4g | 2-(4,5-NO2)-Thiophene | CH3 | 5,000 | >30,000 | >10,000 | >6.0 | >2.0 |
 | 4h | 3-(4-Br)-Thiophene | CH3 | 4.02 ± 0.34 | 183 ± 69 | >10,000 | 46 | >2,488 |
 | 5a | 2-Thiophene | H | 0.11 ± 0.006 | 12.2 ± 0.9 | 75.3 ± 9.6 | 111 | 685 |
 | 5b | 3-Thiophene | H | 0.23 ± 0.02 | 6.4 ± 0.27 | 39 ± 0.8 | 28 | 170 |
(3′,4′-Disubstituted phenyl)-tropanes
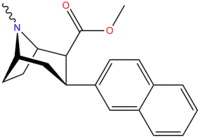


| Compound (+ S. Singh's name) | X (4′-para) | Y (3′-meta) | 2 Position | config | 8 | DA | 5-HT | NE |
|---|---|---|---|---|---|---|---|---|
| RTI-318 11bb | β-naphthyl | CO2Me | β,β | NMe | 0.5 | 0.81 | 20 | |
| Dichloropane (RTI-111ɑ)[10] 17c | Cl | Cl | CO2Me | β,β | NMe | 0.79 | 3.13 | 18.0 |
| RTI-88 [recheck] 17e | NH2 | I | CO2Me | β,β | NMe | 1.35 | 1329c | 320c |
| RTI-97 17d | NH2 | Br | CO2Me | β,β | NMe | 3.91 | 181 | 282 |
| RTI-112b 17b | Cl | Me | CO2Me | β,β | NMe | 0.82 | 10.5 | 36.2 |
| RTI-96 17a | F | Me | CO2Me | β,β | NMe | 2.95 | 76 | 520 |
| RTI-295 | Et | I | CO2Me | β,β | NMe | 21.3 | 2.96 | 1349 |
| RTI-353 (EINT) | Et | I | CO2Me | β,β | NH | 331 | 0.69 | 148 |
| RTI-279 | Me | I | CO2Me | β,β | NH | 5.98 | 1.06 | 74.3 |
| RTI-280 | Me | I | CO2Me | β,β | NMe | 3.12 | 6.81 | 484 |
| Meltzer[11] | catechol | CO2Me | β,β | NMe | >100 | ? | ? | |
| Meltzer[11] | OAc | OAc | CO2Me | β,β | NMe | ? | ? | ? |
- ɑas ·HCl (salt)
- bas ·HCl·2 H2O (salt)
- cSingh gives the reverse value with respect to i.e. 1,329 for NET & 320 for 5-HT
Compound 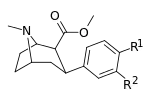 |
Short Name (S. Singh) |
R2 | R1 | DA | 5HT | NE | Selectivity 5-HTT/DAT |
Selectivity NET/DAT |
|---|---|---|---|---|---|---|---|---|
 | meta-fluorophenyltropane 16a | F | H | 23 ± 7.8 | - | - | - | - |
 | meta-chlorophenyltropane 16b | Cl | H | 10.6 ± 1.8 | - | - | - | - |
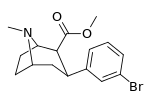 | meta-bromophenyltropane 16c | Br | H | 7.93 ± 0.08ɑ | - | - | - | - |
 | meta-iodophenyltropane 16d | I | H | 26.1 ± 1.7 | - | - | - | - |
 | meta-tributylstannylphenyltropane 16e | SnBu3 | H | 1100 ± 170 | - | - | - | - |
-3-(3-ethynylphenyl)-8-methyl-8-azabicyclo(3.2.1)octane-2-carboxylate.svg.png.webp) | meta-ethynylphenyltropane[3] | C≡CH | H | - | - | - | - | - |
 | meta-methyl-para-fluorophenyltropane RTI-96 17a | CH3 | F | 2.95 ± 0.58 | - | - | - | - |
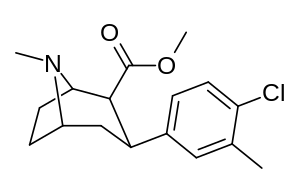
 | meta-methyl-para-chlorophenyltropane RTI-112c 17b | CH3 | Cl | 0.81 ± 0.05 | 10.5 ± 0.05 | 36.2 ± 1.0 | 13.0 | 44.7 |
 | meta-para-dichlorophenyltropane RTI-111b[10] Dichloropane 17c | Cl | Cl | 0.79 ± 0.08b | 3.13 ± 0.36b | 18.0 ± 0.8 17.96 ± 0.85'b'd | 4.0b | 22.8b |
 | meta-bromo-para-aminophenyltropane RTI-97 17d | Br | NH2 | 3.91 ± 0.59 | 181 | 282 | 46.2 | 72.1 |
 | meta-iodo-para-aminophenyltropane RTI-88 17e | I | NH2 | 1.35 ± 0.11 | 120 ± 4 | 1329 ± 124 | 88.9 | 984 |
 | meta-iodo-para-azidophenyltropane 17f | I | N3 | 4.93 ± 0.32 | - | - | - | - |
- ɑIC50 determined in Cynomolgous monkey caudate-putamen
- bas ·HCl (salt)
- cas ·HCl·2 H2O (salt)
- dNEN
Structure 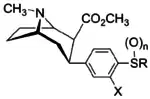 |
Compound | R | X | n | Inhibition of [3H]WIN 35,428 @ DAT IC50 (nM) |
Inhibition of [3H]Paroxetine @ 5-HTT Ki (nM) |
Inhibition of [3H]Nisoxetine @ NET Ki (nM) |
NET/DAT (uptake ratio) |
NET/5-HTT (uptake ratio) |
|---|---|---|---|---|---|---|---|---|---|
| Cocaine | Des-thio/sulfinyl/sulfonyl H | H | Desmethyl 0 | 89.1 | 95 | 1990 | 22 | 21 | |
| para-methoxyphenyltropane Singh: 11i | Des-thio/sulfinyl/sulfonyl OCH3 | H | 0 | 6.5 ± 1.3 | 4.3 ± 0.5 | 1110 ± 64 | 171 | 258 | |
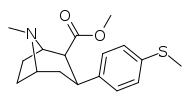 | 7a | CH3 | H | 0 | 9 ± 3 | 0.7 ± 0.2 | 220 ± 10 | 24 | 314 |
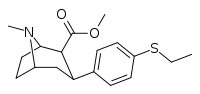 | 7b | C2H5 | H | 0 | 232 ± 34 | 4.5 ± 0.5 | 1170 ± 300 | 5 | 260 |
 | 7c | CH(CH3)2 | H | 0 | 16 ± 2 | 23 ± 2 | 129 ± 2 | 8 | 7 |
 | 7d | CF3 | H | 0 | 200 ± 70 | 8 ± 2 | 1900 ± 300 | 10 | 238 |
 | 7e | CH3 | Br | 0 | 10.1 ± 1 | 0.6 ± 0.2 | 121 ± 12 | 12 | 202 |
 | 7f | CH3 | Br | 1 | 76 ± 18 | 3.2 ± 0.4 | 690 ± 80 | 9 | 216 |
 | 7g | CH3 | H | 1 | 91 ± 16 | 4.3 ± 0.6 | 515 ± 60 | 6 | 120 |
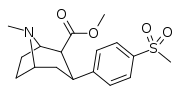 | 7h | CH3 | H | 2 | >10,000 | 208 ± 45 | >10,000 | 1 | 48 |
(2′,4′-Disubstituted phenyl)-tropanes
Compound structure |
Trivial IUPAC (non-systematic) Name |
R2 ortho |
R1 para |
DA | 5HT | NE | Selectivity 5-HTT/DAT |
Selectivity NET/DAT |
|---|---|---|---|---|---|---|---|---|
 | ortho,para-dinitrophenyltropane[13] | NO2 | NO2 | - | - | - | - | - |
(3′,4′,5′-Trisubstituted para-methoxyphenyl)-tropanes
Structure |
Short Name (All compounds tested as HCl salts) |
R2 3′-(meta) |
R3 5′-(di-meta) |
OR1 4′-(para) |
DAT IC50 [3H](compound #)12 |
5-HTT Ki [3H]Paroxetine |
NET Ki [3H]Nisoxetine |
Selectivity NET/DAT Ratio Ki/IC50 |
Selectivity NET/5-HTT Ratio Ki/Ki |
|---|---|---|---|---|---|---|---|---|---|
| Cocaine | - | - | - | 89.1 | 95 | 1990 | 22 | 21 | |
| 6 RTI-112 | - | - | - | 0.82 ± 0.05 | 0.95 ± 0.04 | 21.8 ± 0.6 | 27 | 23 | |
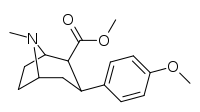 | 7a 11i | H | H | CH3 | 6.5 ± 1.3 | 4.3 ± 0.5 | 1110 ± 64 | 171 | 258 |
 | 7b | H | H | C2H5 | 92 ± 8 | 1.7 ± 0.4 | 1690 ± 50 | 18 | 994 |
 | 7c | F | H | CH3 | 16 ± 1 | 4.8 ± 0.5 | 270 ± 50 | 17 | 56 |
 | 7d | Br | H | CH3 | 47 ± 15 | 3.1 ± 0.1 | 160 ± 20 | 3 | 52 |
 | 7f | Br | Br | CH3 | 92 ± 22 | 2.9 ± 0.1 | 4100 ± 400ɑ | 45 | 1413 |
 | 7e | I | H | CH3 | 170 ± 60 | 3.5 ± 0.4 | 180 ± 20 | 1 | 51 |
 | 7g | I | I | CH3 | 1300 ± 200 | 7.5 ± 0.8 | 180 ± 20 | 4 | 667 |
ɑN=2
(2′,4′,5′-Trisubstituted phenyl)-tropanes
| Structure | Short Name | R1 2′-(ortho) |
R2 4′-(para) |
R3 5′-(meta) |
DAT | 5-HTT | NET | Selectivity NET/DAT Ratio |
Selectivity NET/5-HTT Ratio |
|---|---|---|---|---|---|---|---|---|---|
-3-(4-ethyl-2%252C5-diiodophenyl)-8-methyl-8-azabicyclo(3.2.1)octane-2-carboxylate.svg.png.webp) | para-ethyl-ortho, meta-diiodophenyltropane[3] | iodo | ethyl | iodo | - | - | - | - | - |
2-Carbmethoxy modified (replaced/substituted)
General 2-carbmethoxy modifications
2β-substitutions of p-methoxy-phenyltropanes
Structure |
Short Name (All compounds tested as HCl salts) |
CO2R (2β-substituted) (compound 9 is 2β=R) |
DAT IC50 [3H](compound #)12 |
5-HTT Ki [3H]Paroxetine |
NET Ki [3H]Nisoxetine |
Selectivity NET/DAT Ratio Ki/IC50 |
Selectivity NET/5-HTT Ratio Ki/Ki |
|---|---|---|---|---|---|---|---|
 | 7a 11i | CH3 | 6.5 ± 1.3 | 4.3 ± 0.5 | 1110 ± 64 | 171 | 258 |
 | 8a | (CH3)2CH | 14 ± 3 | 135 ± 35 | 2010 ± 200 | 144 | 15 |
 | 8b | cyclopropane | 6.0 ± 2 | 29 ± 3 | 1230 ± 140 | 205 | 42 |
 | 8c | cyclobutane | 13 ± 3 | 100 ± 8 | >3000 | 231 | 30 |
 | 8d | O2N...1,4-xylene...(CH2)2 | 42 ± 8 | 2.9 ± 0.2 | 330 ± 20 | 8 | 114 |
 | 8e | H2N...1,4-xylene...(CH2)2 | 7.0 ± 2 | 8.3 ± 0.4 | 2200 ± 300ɑ | 314 | 265 |
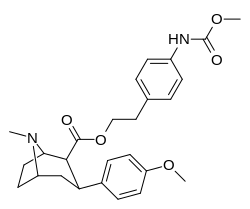 | 8f | CH3CONH...1,4-xylene...(CH2)2 | 6.0 ± 1 | 5.5 ± 0.5 | 1460 ± 30 | 243 | 265 |
 | 8g | H2N...2-bromo-1,4-dimethylbenzene...(CH2)2 | 3.3 ± 1.4 | 4.1 ± 0.6 | 1850 ± 90 | 561 | 451 |
 | 8h | H2N...1,3-dibromo-2,5-dimethylbenzene...(CH2)2 | 15 ± 6 | 2.0 ± 0.4 | 2710 ± 250ɑ | 181 | 1360 |
 | 8i | H2N...2-iodo-1,4-dimethylbenzene...(CH2)2 | 2.5 ± 0.7 | 3.5 ± 1 | 2040 ± 300ɑ | 816 | 583 |
 | 8j | H2N...1,3-diiodo-2,5-dimethylbenzene...(CH2)2 | 102 ± 15 | 1.0 ± 0.1 | 2600 ± 200ɑ | 25 | 2600 |
 | 9 | 3-(4-methylphenyl)-1,2-oxazole | 18 ± 6 | 860 ± 170 | >3000 | 167 | 3 |
ɑN=2
2β-carboxy side-chained (p-chloro/iodo/methyl) phenyltropanes
Compound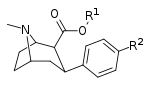 |
Short Name (S. Singh) |
R | X | IC50 (nM) DAT [3H]WIN 35428 |
IC50 (nM) 5-HTT [3H]paroxetine |
IC50 (nM) NET [3H]nisoxetine |
Selectivity 5-HTT/DAT |
Selectivity NET/DAT |
|---|---|---|---|---|---|---|---|---|
 | 23a | CH(CH3)2 | H | 85.1 ± 2.5 | 23121 ± 3976 | 32047 ± 1491 | 272 | 376 |
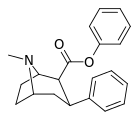 | 23b | C6H5 | H | 76.7 ± 3.6 | 106149 ± 7256 | 19262 ± 593 | 1384 | 251 |
 | 24a | CH(CH3)2 | Cl | 1.4 ± 0.13 6.04 ± 0.31ɑ | 1400 ± 7 128 ± 15b | 778 ± 21 250 ± 0.9c | 1000 21.2d | 556 41.4e |
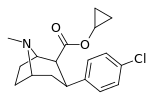 | 24b | cyclopropyl | Cl | 0.96 ± 0.10 | 168 ± 1.8 | 235 ± 8.39 | 175 | 245 |
 | 24c | C6H5 | Cl | 1.99 ± 0.05 5.25 ± 0.76ɑ | 2340 ± 27 390 ± 34b | 2960 ± 220 242 ± 30c | 1176 74.3d | 1.3 41.6e |
 | 24d | C6H4-4-I | Cl | 32.6 ± 3.9 | 1227 ± 176 | 967.6 ± 26.3 | 37.6 | 29.7 |
 | 24e | C6H4-3-CH3 | Cl | 9.37 ± 0.52 | 2153 ± 143 | 2744 ± 140 | 230 | 293 |
 | 24f | C6H4-4-CH3 | Cl | 27.4 ± 1.5 | 1203 ± 42 | 1277 ± 118 | 43.9 | 46.6 |
 | 24g | C6H4-2-CH3 | Cl | 3.91 ± 0.23 | 3772 ± 384 | 4783 ± 387 | 965 | 1223 |
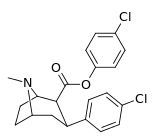 | 24h | C6H4-4-Cl | Cl | 55 ± 2.3 | 16914 ± 1056 | 4883 ± 288 | 307 | 88.8 |
 | 24i | C6H4-4-OCH3 | Cl | 71 ± 5.6 | 19689 ± 1843 | 1522 ± 94 | 277 | 21.4 |
 | 24j | (CH2)2C6H4-4-NO2 | Cl | 2.71 ± 0.13 | - | - | - | - |
 | 24k | (CH)2C6H4-4-NH2 | Cl | 2.16 ± 0.25 | - | - | - | - |
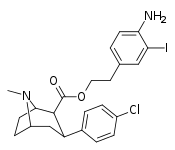 | 24l | (CH2)2C6H3-3-I-4-NH2 | Cl | 2.51 ± 0.25 | - | - | - | - |
 | 24m | (CH2)2C6H3-3-I-4-N3 | Cl | 14.5 ± 0.94 | - | - | - | - |
 | 24n | (CH2)2C6H4-4-N3 | Cl | 6.17 ± 0.57 | - | - | - | - |
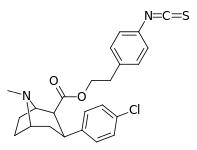 | 24o | (CH2)2C6H4-4-NCS | Cl | 5.3 ± 0.6 | - | - | - | - |
 | 24p | (CH2)2C6H4-4-NHCOCH2Br | Cl | 1.73 ± 0.06 | - | - | - | - |
 | 25a | CH(CH3)2 | I | 0.43 ± 0.05 2.79 ± 0.13ɑ | 66.8 ± 6.53 12.5 ± 1.0b | 285 ± 7.6 41.2 ± 3.0c | 155 4.5d | 663 14.8e |
 | 25b | cyclopropyl | I | 0.61 ± 0.08 | 15.5 ± 0.72 | 102 ± 11 | 25.4 | 167 |
 | 25c | C6H5 | I | 1.51 ± 0.34 6.85 ± 0.93ɑ | 184 ± 22 51.6 ± 6.2b | 3791 ± 149 32.7 ± 4.4c | 122 7.5d | 2510 4.8e |
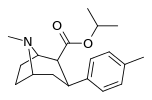 | 26a | CH(CH3)2 | CH3 | 6.45 ± 0.85 15.3 ± 2.08ɑ | 6090 ± 488 917 ± 54b | 1926 ± 38 73.4 ± 11.6c | 944 59.9d | 299 4.8e |
 | 26b | CH(C2H5)2 | CH3 | 19.1 ± 1 | 4499 ± 557 | 3444 ± 44 | 235 | 180 |
 | 26c | cyclopropyl | CH3 | 17.8 ± 0.76 | 485 ± 21 | 2628 ± 252 | 27.2 | 148 |
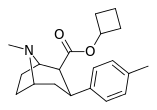 | 26d | cyclobutyl | CH3 | 3.74 ± 0.52 | 2019 ± 133 | 4738 ± 322 | 540 | 1267 |
 | 26e | cyclopentyl | CH3 | 1.68 ± 0.14 | 1066 ± 109 | 644 ± 28 | 634 | 383 |
 | 26f | C6H5 | CH3 | 3.27 ± 0.06 9.13 ± 0.79ɑ | 24500 ± 1526 1537 ± 101b | 5830 ± 370 277 ± 23c | 7492 168d | 1783 30.3e |
 | 26g | C6H4-3-CH3 | CH3 | 8.19 ± 0.90 | 5237 ± 453 | 2136 ± 208 | 639 | 261 |
 | 26h | C6H4-4-CH3 | CH3 | 81.2 ± 16 | 15954 ± 614 | 4096 ± 121 | 196 | 50.4 |
 | 26i | C6H4-2-CH3 | CH3 | 23.2 ± 0.97 | 11040 ± 504 | 25695 ± 1394 | 476 | 1107 |
 | 26j | C6H4-4-Cl | CH3 | 117 ± 7.9 | 42761 ± 2399 | 9519 ± 864 | 365 | 81.3 |
 | 26k | C6H4-4-OCH3 | CH3 | 95.6 ± 8.8 | 82316 ± 7852 | 3151 ± 282 | 861 | 33.0 |
- ɑKi value for displacement of [3H]DA uptake.
- bKi value for displacement of [3H]5-HT uptake.
- cKi value for displacement of [3H]NE uptake.
- d[3H]5-HT uptake to [3H]DA uptake ratio.
- e[3H]NE uptake to [3H]DA uptake ratio.
Carboxyaryl
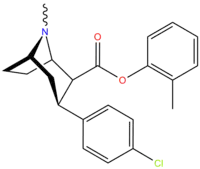


| Compound | X | 2 Position | config | 8 | DA | 5-HT | NE |
|---|---|---|---|---|---|---|---|
| RTI-122 | I | -CO2Ph | β,β | NMe | 1.50 | 184 | 3,791 |
| RTI-113 | Cl | -CO2Ph | β,β | NMe | 1.98 | 2,336 | 2,955 |
| RTI-277 | NO2 | -CO2Ph | β,β | NMe | 5.94 | 2,910 | 5,695 |
| RTI-120 [recheck] | Me | -CO2Ph | β,β | NMe | 3.26 | 24,471 | 5,833 |
| RTI-116 | Cl | -CO2(p-C6H4I) | β,β | NMe | 33 | 1,227 | 968 |
| RTI-203 | Cl | CO2(m-C6H4Me) | β,β | NMe | 9.37 | 2153 | 2744 |
| RTI-204 | Cl | -CO2(o-C6H4Me) | β,β | NMe | 3.91 | 3,772 | 4,783 |
| RTI-205 | Me | -CO2(m-C6H4Me) | β,β | NMe | 8.19 | 5,237 | 2,137 |
| RTI-206 | Cl | -CO2(p-C6H4Me) | β,β | NMe | 27.4 | 1,203 | 1,278 |
2-Phenyl-3-Phenyltropanes
| Compound Structure | Short Name (S. Singh) |
Stereochemistry | X (para) |
DAT [3H]WIN 35428 IC50 (nM) |
DAT [3H]Mazindol Ki (nM) |
5-HTT [3H]Paroxetine IC50 (nM) |
[3H]DA uptake Ki (nM) | [3H]5-HT uptake Ki (nM) | Selectivity [3H]5-HT/[3H]DA |
|---|---|---|---|---|---|---|---|---|---|
| Cocaine | (2β,3β) | (H) | 89 ± 4.8 | 281 | 1050 ± 89 | 423 | 155 | 0.4 | |
 | 67a | 2β,3β | H | 12.6 ± 1.8 | 14.9 | 21000 ± 3320 | 28.9 | 1100 | 38.1 |
 | 67b | 2β,3α | H | - | 13.8 | - | 11.7 | 753 | 64.3 |
 | 67c | 2α,3α | H | 690 ± 37 | - | 41300 ± 5300 | - | - | - |
 | 68 | 2β,3α | F | - | 6.00 | - | 4.58 | 122 | 26.6 |
 | 69a | 2β,3β | CH3 | 1.96 ± 0.08 | 2.58 | 11000 ± 83 | 2.87 | 73.8 | 25.7 |
 | 69b | 2β,3α | CH3 | - | 2.87 | - | 4.16 | 287 | 69.0 |
 | 69c | 2α,3α | CH3 | 429 ± 59 | - | 15800 ± 3740 | - | - | - |
Carboxyalkyl


| Code | X | 2 Position | config | 8 | DA | 5-HT | NE |
|---|---|---|---|---|---|---|---|
| RTI-77 | Cl | CH2C2(3-iodo-p-anilino) | β,β | NMe | 2.51 | — | 2247 |
| RTI-121 IPCIT | I | -CO2Pri | β,β | NMe | 0.43 | 66.8 | 285 |
| RTI-153 | I | -CO2Pri | β,β | NH | 1.06 | 3.59 | 132 |
| RTI-191 | I | -CO2Prcyc | β,β | NMe | 0.61 | 15.5 | 102 |
| RTI-114 | Cl | -CO2Pri | β,β | NMe | 1.40 | 1,404 | 778 |
| RTI-278 | NO2 | -CO2Pri | β,β | NMe | 8.14 | 2,147 | 4,095 |
| RTI-190 | Cl | -CO2Prcyc | β,β | NMe | 0.96 | 168 | 235 |
| RTI-193 | Me | -CO2Prcyc | β,β | NMe | 1.68 | 1,066 | 644 |
| RTI-117 | Me | -CO2Pri | β,β | NMe | 6.45 | 6,090 | 1,926 |
| RTI-150 | Me | -CO2Bucyc | β,β | NMe | 3.74 | 2,020 | 4,738 |
| RTI-127 | Me | -CO2C(H)Et2 | β,β | NMe | 19 | 4500 | 3444 |
| RTI-338 | ethyl | -CO2C2Ph | β,β | NMe | 1104 | 7.41 | 3366 |
Use of a cyclopropyl ester appears to enable better MAT retention than does the choice of isopropyl ester.
Use of a cycBu resulted in greater DAT selectivity than did the cycPr homologue.
2-Alkyl Esters & Ethers
Esters (2-Alkyl)
| Structure | Short Name (S. Singh) |
2β=R | Ki (nM) DAT [3H]WIN 35428 |
IC50 (nM) [3H]DA uptake |
Selectivity uptake/binding |
|---|---|---|---|---|---|
 | 59a | CH=CHCO2CH3 | 22 ± 2 | 123 ± 65 | 5.6 |
 | 59b | CH2CH2CO2CH3 | 23 ± 2 | 166 ± 68 | 7.2 |
 | 59c | (CH2)2CH=CHCO2CH3 | 20 ± 2 | 203 ± 77 | 10.1 |
 | 59d | (CH22)4CO2CH3 | 30 ± 2 | 130 ± 7 | 4.3 |
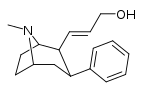 | 59e | CH=CHCH2OH | 26 ± 3 | 159 ± 43 | 6.1 |
 | 59f | CH2CH2CH2OH | 11 ± 1 | 64 ± 32 | 5.8 |
 | 59g | CH2CH2COC6H5 | 28 ± 2 | 47 ± 15 | 1.7 |
Ethers (2-Alkyl)
See the N-desmethyl Paroxetine homologues
| Molecular Structure | Short Name (S. Singh) |
Stereochemistry | DAT [3H]WIN 35428 IC50 (nM) |
5-HTT [3H]Paroxetine IC50 (nM) |
NET [3H]Nisoxetine IC50 (nM) |
Selectivity 5-HTT/DAT |
Selectivity NET/DAT |
|---|---|---|---|---|---|---|---|
| Paroxetine | 623 ± 25 | 0.28 ± 0.02 | 535 ± 15 | 0.0004 | 0.8 | ||
 | R-60a | 2β,3β | 308 ± 20 | 294 ± 18 | 5300 ± 450 | 0.9 | 17.2 |
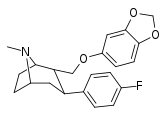 | R-60b | 2α,3β | 172 ± 8.8 | 52.9 ± 3.6 | 26600 ± 1200 | 0.3 | 155 |
 | R-60c | 2β,3α | 3.01 ± 0.2 | 42.2 ± 16 | 123 ± 9.5 | 14.1 | 40.9 |
 | S-60d | 2β,3β | 1050 ± 45 | 88.1 ± 2.8 | 27600 ± 1100 | 0.08 | 26.3 |
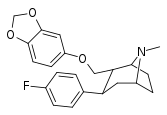 | S-60e | 2α,3β | 1500 ± 74 | 447 ± 47 | 2916 ± 1950 | 0.3 | 1.9 |
 | S-60f | 2β,3α | 298 ± 17 | 178 ± 13 | 12400 ± 720 | 0.6 | 41.6 |
Carboxamides
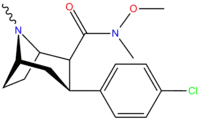


Structure 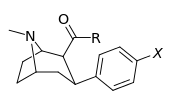 | Code (S. Singh #) | X | 2 Position | config | 8 | DA [3H]WIN 35428 (IC50 nM) | NE [3H]nisoxetine | 5-HT [3H]paroxetine (IC50 nM) | Selectivity 5-HTT/DAT | Selectivity NET/DAT |
|---|---|---|---|---|---|---|---|---|---|---|
 | RTI-106 27b | Cl | CON(H)Me | β,β | NMe | 12.4 ± 1.17 | 1584 ± 62 | 1313 ± 46 | 106 | 128 |
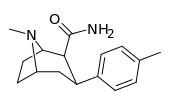
 | RTI-118 27a | Cl | CONH2 | β,β | NMe | 11.5 ± 1.6 | 4270 ± 359 | 1621 ± 110 | 141 | 371 |
 | RTI-222 29d | Me | morpholinyl | β,β | NMe | 11.7 ± 0.87 | 23601 ± 1156 | >100K | >8547 | 2017 |
 | RTI-129 27e | Cl | CONMe2 | β,β | NMe | 1.38 ± 0.1 | 942 ± 48 | 1079 ± 102 | 792 | 683 |
 | RTI-146 27d | Cl | CONHCH2OH | β,β | NMe | 2.05 ± 0.23 | 144 ± 3 | 97.8 ± 10 | 47.7 | 70.2 |
 | RTI-147 27i | Cl | CON(CH2)4 | β,β | NMe | 1.38 ± 0.03 | 3,950 ± 72 | 12400 ± 1207 | 8985 | 2862 |
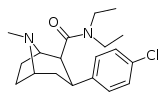 | RTI-156 | Cl | CON(CH2)5 | β,β | NMe | 6.61 | 5832 | 3468 | ||
 | RTI-170 | Cl | CON(H)CH2C≡CH | β,β | NMe | 16.5 | 1839 | 4827 | ||
 | RTI-172 | Cl | CON(H)NH2 | β,β | NMe | 44.1 | 3914 | 3815 | ||
 | RTI-174 | Cl | CONHCOMe | β,β | NMe | 158 | >43K | >125K | ||
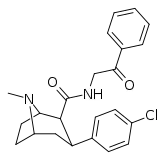 | RTI-182 | Cl | CONHCH2COPh | β,β | NMe | 7.79 | 1722 | 827 | ||
 | RTI-183✲ 27 g | Cl | CON(OMe)Me | β,β | NMe | 0.85 ± 0.06 | 549 ± 18.5 | 724 ± 94 | 852 | 646 |
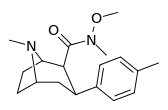 | RTI-186 29c | Me | CON(OMe)Me | β,β | NMe | 2.55 ± 0.43 | 422 ± 26 | 3402 ± 353 | 1334 | 165 |
 | RTI-198 27h | Cl | CON(CH2)3 | β,β | NMe | 6.57 ± 0.67 | 990 ± 4.8 | 814 ± 57 | 124 | 151 |
 | RTI-196 27c | Cl | CONHOMe | β,β | NMe | 10.7 ± 1.25 | 9907 ± 632 | 43700 ± 1960 | 4084 | 926 |
 | RTI-201 | Cl | CONHNHCOPh | β,β | NMe | 91.8 | >20K | >48K | ||
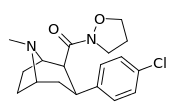 | RTI-208 27j | Cl | CONO(CH2)3 | β,β | NMe | 1.47 ± 0.13 | 1083 ± 76 | 2470 ± 56 | 1680 | 737 |
 | RTI-214 27l | Cl | CON(-CH2CH2-)2O | β,β | NMe | 2.90 ± 0.3 | 8545 ± 206 | 88769 ± 1855 | 30610 | 2946 |
 | RTI-215 27f | Cl | CONEt2 | β,β | NMe | 5.48 ± 0.19 | 5532 ± 299 | 9433 ± 770 | 1721 | 1009 |
 | RTI-217 | Cl | CONH(m-C6H4OH) | β,β | NMe | 4.78 | >30K | >16K | ||
 | RTI-218✲ | Cl | CON(Me)OMe | β,β | NMe | 1.19 | 520 | 1911 | ||
 | RTI-226 27 m | Cl | CONMePh | β,β | NMe | 45.5 ± 3 | 2202 ± 495 | 23610 ± 2128 | 519 | 48.4 |
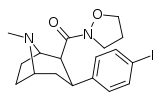 | RTI-227 | I | CONO(CH2)3 | β,β | NMe | 0.75 | 446 | 230 | ||
 | RTI-229[16] 28a | I | CON(CH2)4 | β,β | NMe | 0.37 ± 0.04 | 991 ± 21 | 1728 ± 39 | 4670 | 2678 |
 | 27k | 6.95 ± 1.21 | 1752 ± 202 | 3470 ± 226 | 499 | 252 | ||||
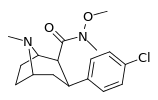 | 28b | 1.08 ± 0.15 | 103 ± 6.2 | 73.9 ± 8.1 | 68.4 | 95.4 | ||||
 | 28c | 0.75 ± 0.02 | 357 ± 42 | 130 ± 15.8 | 173 | 476 | ||||
 | 29a | 41.8 ± 2.45 | 4398 ± 271 | 6371 ± 374 | 152 | 105 | ||||
 | 29b | 24.7 ± 1.93 | 6222 ± 729 | 33928 ± 2192 | 1374 | 252 |
✲RTI-183 and RTI-218 suggest possible copy-error, seeing as "CON(OMe)Me" & "CON(Me)OMe" difference between methyl & methoxy render as the same.
| Compound | Short Name (S. Singh) |
R | X | IC50 (nM) DAT [3H]WIN 35428 |
IC50 (nM) 5-HTT [3H]Paroxetine |
IC50 (nM) NET [3H]Nisoxetine |
Selectivity 5-HTT/DAT |
Selectivity NET/DAT |
|---|---|---|---|---|---|---|---|---|
 | ||||||||
| 29a | NH2 | CH3 | 41.8 ± 2.45 | 6371 ± 374 | 4398 ± 271 | 152 | 105 | |
| 29b | N(CH2CH3)2 | CH3 | 24.7 ± 1.93 | 33928 ± 2192 | 6222 ± 729 | 1374 | 252 | |
| 29c RTI-186 | N(OCH3)CH3 | CH3 | 2.55 ± 0.43 | 3402 ± 353 | 422 ± 26 | 1334 | 165 | |
| 29d RTI-222 | 4-morpholine | CH3 | 11.7 ± 0.87 | >100000 | 23601 ± 1156 | >8547 | 2017 |
Carboxamide linked phenyltropanes dimers

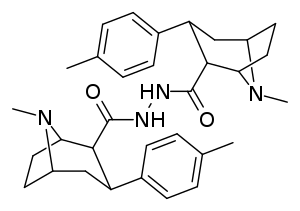


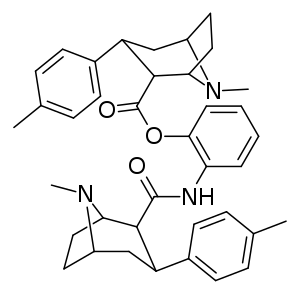
Dimers of phenyltropanes, connected in their dual form using the C2 locant as altered toward a carboxamide structural configuring (in contrast and away from the usual inherent ecgonine carbmethoxy), as per Frank Ivy Carroll's patent inclusive of such chemical compounds, possibly so patented due to being actively delayed pro-drugs in vivo.[3]
Heterocycles
These heterocycles are sometimes referred to as the "bioisosteric equivalent" of the simpler esters from which they are derived. A potential disadvantage of leaving the ββ-ester unreacted is that in addition to being hydrolyzable, it can also epimerize[17] to the energetically more favorable trans configuration. This can happen to cocaine also.

(compound model 34)
Several of the oxadiazoles contain the same number and types of heteroatoms, while their respective binding potencies display 8×-15× difference. A finding that would not be accounted for by their affinity originating from hydrogen bonding.
To explore the possibility of electrostatic interactions, the use of molecular electrostatic potentials (MEP) were employed with model compound 34 (replacing the phenyltropane moiety with a methyl group). Focusing on the vicinity of the atoms @ positions A—C, the minima of electrostatic potential near atom position A (ΔVmin(A)), calculated with semi-empirical (AM1) quantum mechanics computations (superimposing the heterocyclic and phenyl rings to ascertain the least in the way of steric and conformational discrepancies) found a correlation between affinity @ DAT and ΔVmin(A): wherein the values for the latter for 32c = 0, 32g = -4, 32h = -50 & 32i = -63 kcal/mol.
In contrast to this trend, it is understood that an increasingly negative ΔVmin is correlated with an increase of strength in hydrogen bonding, which is the opposing trend for the above; this indicates that the 2β-substituents (at least for the heterocyclic class) are dominated by electrostatic factors for binding in-the-stead of the presumptive hydrogen bonding model for this substituent of the cocaine-like binding ligand.[lower-alpha 7]
3-Substituted-isoxazol-5-yl

| Code (S.S. #) |
X | R | DA | NE | 5HT |
|---|---|---|---|---|---|
| RTI-165 | Cl | 3-methylisoxazol-5-yl | 0.59 | 181 | 572 |
| RTI-171 | Me | 3-methylisoxazol-5-yl | 0.93 | 254 | 3818 |
| RTI-180 | I | 3-methylisoxazol-5-yl | 0.73 | 67.9 | 36.4 |
| RTI-177 β-CPPIT 32g | Cl | 3-phenylisoxazol-5-yl | 1.28 ± 0.18 | 504 ± 29 | 2420 ± 136 |
| RTI-176 | Me | 3-phenylisoxazol-5-yl | 1.58 | 398 | 5110 |
| RTI-181 | I | 3-phenylisoxazol-5-yl | 2.57 | 868 | 100 |
| RTI-184 | H | methyl | 43.3 | — | 6208 |
| RTI-185 | H | Ph | 285 | — | >12K |
| RTI-334 | Cl | 3-ethylisoxazol-5-yl | 0.50 | 120 | 3086 |
| RTI-335 | Cl | isopropyl | 1.19 | 954 | 2318 |
| RTI-336 | Cl | 3-(4-methylphenyl)isoxazol-5-yl | 4.09 | 1714 | 5741 |
| RTI-337 | Cl | 3-t-butyl-isoxazol-5-yl | 7.31 | 6321 | 37K |
| RTI-345 | Cl | p-chlorophenyl | 6.42 | 5290 | >76K |
| RTI-346 | Cl | p-anisyl | 1.57 | 762 | 5880 |
| RTI-347 | Cl | p-fluorophenyl | 1.86 | 918 | 7257 |
| RTI-354 | Me | 3-ethylisoxazol-5-yl | 1.62 | 299 | 6400 |
| RTI-366 | Me | R = isopropyl | 4.5 | 2523 (1550) | 42,900 (3900) |
| RTI-371 | Me | p-chlorophenyl | 8.74 | >100K (60,200) | >100K (9090) |
| RTI-386 | Me | p-anisyl | 3.93 | 756 (450) | 4027 (380) |
| RTI-387 | Me | p-fluorophenyl | 6.45 | 917 (546) | >100K (9400) |
3-Substituted-1,2,4-oxadiazole
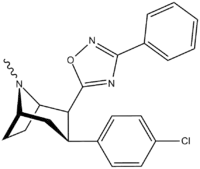
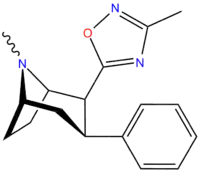
| Structure | Code (Singh's #) |
X | R | DAT (IC50 nM) displacement of [H3]WIN 35428 |
NET (IC50 nM) [H3]nisoxetine |
5-HTT (IC50 nM) [H3]paroxetine |
Selectivity 5-HTT/DAT |
Selectivity NET/DAT |
|---|---|---|---|---|---|---|---|---|
 | ααRTI-87 | H | 3-methyl-1,2,4-oxadiazole | 204 | 36K | 30K | ||
 | βαRTI-119 | H | 3-methyl-1,2,4-oxadiazole | 167 | 7K | 41K | ||
 | αβRTI-124 | H | 3-methyl-1,2,4-oxadiazole | 1028 | 71K | 33K | ||
 | RTI-125 (32a) | Cl | 3-methyl-1,2,4-oxadiazole | 4.05 ± 0.57 | 363 ± 36 | 2584 ± 800 | 637 | 89.6 |
 | ββRTI-126[18] (31) | H | 3-methyl-1,2,4-oxadiazole | 100 ± 6 | 7876 ± 551 | 3824 ± 420 | 38.3 | 788 |
 | RTI-130 (32c) | Cl | 3-phenyl-1,2,4-oxadiazole | 1.62 ± 0.02 | 245 ± 13 | 195 ± 5 | 120 | 151 |
 | RTI-141 (32d) | Cl | 3-(p-anisyl)-1,2,4-oxadiazole | 1.81 ± 0.19 | 835 ± 8 | 337 ± 40 | 186 | 461 |
 | RTI-143 (32e) | Cl | 3-(p-chlorophenyl)-1,2,4-oxadiazole | 4.06 ± 0.22 | 40270 ± 180 (4069) | 404 ± 56 | 99.5 | 9919 |
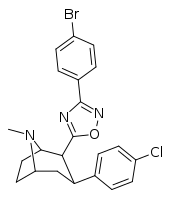 | RTI-144 (32f) | Cl | 3-(p-bromophenyl)-1,2,4-oxadiazole | 3.44 ± 0.36 | 1825 ± 170 | 106 ± 10 | 30.8 | 532 |
 | βRTI-151 (33) | Me | 3-phenyl-1,2,4-oxadiazole | 2.33 ± 0.26 | 60 ± 2 | 1074 ± 130 | 459 | 25.7 |
 | αRTI-152 | Me | 3-phenyl-1,2,4-oxadiazole | 494 | — | 1995 | ||
 | RTI-154 (32b) | Cl | 3-isopropyl-1,2,4-oxadiazole | 6.00 ± 0.55 | 135 ± 13 | 3460 ± 250 | 577 | 22.5 |
 | RTI-155 | Cl | 3-cyclopropyl-1,2,4-oxadiazole | 3.41 | 177 | 4362 |

↑ above: 2D skeletal depiction.
↓ below: 3D tube model.



| Structure | Code | X | 2 Group | DAT (IC50 nM) displacement of [H3]WIN 35428 |
NET (IC50 nM) displacement of [H3]nisoxetine |
5-HTT (IC50 nM) displacement of [H3]paroxetine |
Selectivity 5-HTT/DAT |
Selectivity NET/DAT |
|---|---|---|---|---|---|---|---|---|
 | RTI-157 | Me | tetrazole | 1557 | >37K | >43K | ||
 | RTI-163 | Cl | tetrazole | 911 | — | 5456 | ||
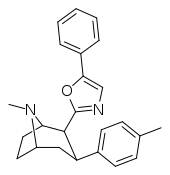 | RTI-178 | Me | 5-phenyl-oxazol-2-yl | 35.4 | 677 | 1699 | ||
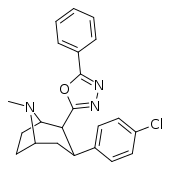 | RTI-188 | Cl | 5-phenyl-1,3,4-oxadiazol-2-yl | 12.6 | 930 | 3304 | ||
 | RTI-189 (32i) | Cl | 5-phenyl-oxazol-2-yl | 19.7 ± 1.98 | 496 ± 42 | 1120 ± 107 | 56.8 | 25.5 |
 | RTI-194 | Me | 5-methyl-1,3,4-oxadiazol-2-yl | 4.45 | 253 | 4885 | ||
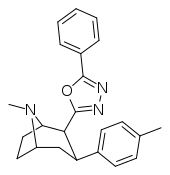 | RTI-195 | Me | 5-phenyl-1,3,4-oxadiazol-2-yl | 47.5 | 1310 | >22,000 | ||
 | RTI-199 | Me | 5-phenyl-1,3,4-thiadiazol-2-yl | 35.9 | >24,000 | >51,000 | ||
 | RTI-200 | Cl | 5-phenyl-1,3,4-thiadiazol-2-yl | 15.3 | 4142 | >18,000 | ||
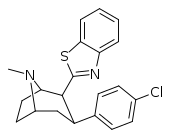 | RTI-202 | Cl | benzothiazol-2-yl | 1.37 | 403 | 1119 | ||
 | RTI-219 | Cl | 5-phenylthiazol-2-yl | 5.71 | 8516 | 10,342 | ||
| RTI-262 | Cl | 188.2 ± 5.01 | 595.25 ± 5738 | 5207 ± 488 | 316 | 28 | ||
 | RTI-370 | Me | 3-(p-cresyl)isoxazol-5-yl | 8.74 | 6980 | >100K | ||
 | RTI-371 | Cl | 3-(p-chlorophenyl)isoxazol-5-yl | 13 | >100K | >100K | ||
 | RTI-436 | Me | -CH=CHPh[20] | 3.09 | 1960 (1181) | 335 (31) | ||
 | RTI-470 | Cl | o-Cl-benzothiazol-2-yl | 0.094 | 1590 (994) | 1080 (98) | ||
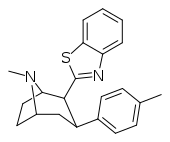 | RTI-451 | Me | benzothiazol-2-yl | 1.53 | 476 (287) | 7120 (647) | ||
 | 32g | 1.28 ± 0.18 | 504 ± 29 | 2420 ± 136 | 1891 | 394 | ||
 | 32h | 12.6 ± 10.3 | 929 ± 88 | 330 ± 196 | 262 | 73.7 |
N.B There are some alternative ways of making the tetrazole ring however; C.f. the sartan drugs synthesis schemes. Bu3SnN3 is a milder choice of reagent than hydrogen azide (c.f. Irbesartan).
Acyl (C2-propanoyl)
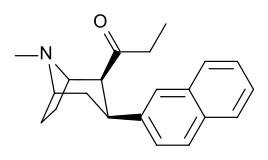
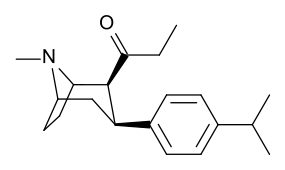


-8-azabicyclo(3.2.1)octanes.png.webp)
cf. the Tamagnan series of phenyltropanes for examples with a methylene unit spacer breaking up the indole.
| # (#) | X | Y | 2 Position | config | 8 | DA | 5-HT | NE |
|---|---|---|---|---|---|---|---|---|
| WF-23 (39n) | β-naphthyl | C(O)Et | β,β | NMe | 0.115 | 0.394 | No data | |
| WF-31 PIT | -Pri | H | C.O.Et | β,β | NMe | 615 | 54.5 | No data |
| WF-11✲ PTT (39e) | Me | H | -C.O.Et | β,β | NMe | 8.2 | 131 | No data |
| WF-25 (39a) | H | H | -C.O.Et | β,β | NMe | 48.3 | 1005 | No data |
| WF-33 | 6-MeoBN | C(O)Et | α,β | NMe | 0.13 | 2.24 | No data | |
| ✲Compound WF-11 has been shown, under consistent exposure, to elicit a biological response opposite of cocaine i.e. tyrosine hydroxylase gene expression down-regulation (instead of up-regulation as has been observed to be the case for chronic cocaine administration) | ||||||||
| Structure | S. Singh's alphanumeric assignation (name) |
R1 | R2 | DAT
[125I]RTI-55 IC50 (nM) |
5-HTT
[3H]Paroxetine Ki (nM) |
Selectivity
5-HTT/DAT |
|---|---|---|---|---|---|---|
| cocaine | 173 ± 19 | — | — | |||
| Troparil 11a (WIN 35065-2) | 98.8 ± 12.2 | — | — | |||
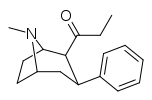 | WF-25 39a | C2H5 | C6H5 | 48.3 ± 2.8 | 1005 ± 112 | 20.8 |
 | 39b | CH3 | C6H5 | 114 ± 22 | 1364 ± 616 | 12.0 |
 | 39c | C2H5 | C6H4-4-F | 15.3 ± 2.8 | 630 ± 67 | 41.2 |
 | 39d | CH3 | C6H4-4-F | 70.8 ± 13 | 857 ± 187 | 12.1 |
 | WF-11 39e | C2H5 | C6H4-4-CH3 | 8.2 ± 1.6 | 131 ± 1 | 16.0 |
| (+)-39e | C2H5 | C6H4-4-CH3 | 4.21 ± 0.05 | 74 ± 12 | 17.6 | |
| (-)-39e | C2H5 | C6H4-4-CH3 | 1337 ± 122 | >10000 | — | |
 | 39f | CH3 | C6H4-4-CH3 | 9.8 ± 0.5 | 122 ± 22 | 12.4 |
 | 39g | CH3 | C6H4-4-C2H5 | 152 ± 24 | 78.2 ± 22 | 0.5 |
 | 39h | C2H5 | C6H4-4-CH(CH3)2 | 436 ± 41 | 35.8 ± 4.4 | 0.08 |
 | 39i | C2H5 | C6H4-4-C(CH3)3 | 2120 ± 630 | 1771 ± 474 | 0.8 |
 | 39j | C2H5 | C6H4-4-C6H5 | 2.29 ± 1.08 | 4.31 ± 0.01 | 1.9 |
 | 39k | C2H5 | C6H4-2-CH3 | 1287 ± 322 | 710000 | >7.8 |
 | 39l | C2H5 | 1-naphthyl | 5.43 ± 1.27 | 20.9 ± 2.9 | 3.8 |
 | 39m | CH3 | 1-naphthyl | 10.1 ± 2.2 | 25.6 ± 5.1 | 2.5 |
 | WF-23 39n | C2H5 | 2-naphthyl | 0.115 ± 0.021 | 0.394 ± 0.074 | 3.5 |
 | 39o | CH3 | 2-naphthyl | 0.28 ± 0.11 | 1.06 ± 0.36 | 3.8 |
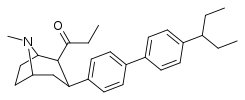 | 39p | C2H5 | C6H4-4-CH(C2H5)2 | 270 ± 38 | 540 ± 51 | 2.0 |
 | 39q | C2H5 | C6H4-4-C6H11 | 320 ± 55 | 97 ± 12 | 0.30 |
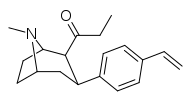 | 39r | C2H5 | C6H4-4-CH=CH2 | 0.90 ± 0.34 | 3.2 ± 1.3 | 3.5 |
 | 39s | C2H5 | C6H4-4-C(=CH2)CH3 | 7.2 ± 2.1 | 0.82 ± 0.38 | 0.1 |
2β-Acyl-3β-naphthyl substituted
| Structure | Short Assignation (Numeric code, Davies UB) S. Singh |
R | DAT [125H]RTI-55ɑ IC50 nM |
SERT [3H]paroxetineb Ki nM |
NET [3H]nisoxetinec Ki nM |
potency ratio SERT/DAT |
potency ratio SERT/NET |
|---|---|---|---|---|---|---|---|
 | WF-11 (6) | 4′-Me | 8.2 ± 1.6 | 131 ± 10 | 65 ± 9.2 | 0.06 | 0.5 |
 | WF-31 (7) | 4′-iPr | 436 ± 41 | 36 ± 4 | >10,000 | 12 | >250 |
 | WF-23 (8) | 2-naphthalene | 0.12 ± 0.02 | 0.39 ± 0.07 | 2.9 ± 0.5 | 0.3 | 7 |
 | 2β-acyl-3β-1-naphthalene (9a) | 4′-H | 5.3 ± 1.3 | 21 ± 2.9 | 49 ± 10 | 0.3 | 18 |
 | (9b) | 4′-Me | 25.1 ± 0.5 | 8.99 ± 1.70 | 163 ± 36 | 3 | 18 |
 | (9c) | 4′-Et | 75.1 ± 11.9 | 175 ± 25 | 4769 ± 688 | 0.7 | 27 |
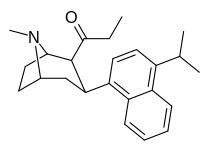 | (9d) | 4′-iPr | 225 ± 36 | 136 ± 64 | >10,000 | 2 | >73.5 |
 | (10a) | 6′-Et | 0.15 ± 0.04 | 0.38 ± 0.19 | 27.7 ± 9.6 | 0.4 | 74 |
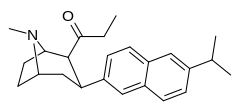 | (10b) | 6′-iPr | 0.39 ± 0.04 | 1.97 ± 0.33 | no data | 0.2 | — |
 | (10ce) | 6′- OMe | 0.13 ± 0.04 | 2.24 ± 0.34 | no data | 0.05 | — |
 | (10d) | 5′-Et, 6′-OMe | 30.8 ± 6.6 | 7.55 ± 1.57 | 3362 ± 148 | 4.1 | 445 |
 | (10e) | 5′-C(Me)=CH2, 6′-OMe | 45.0 ± 3.7 | 88.0 ± 13.3 | 2334 ± 378 | 0.5 | 26.5 |
 | (10f) | 6′-I | 0.35 ± 0.07 | 0.37 ± 0.02 | no data | 1.0 | — |
 | (10g) | 7′-I | 0.45 ± 0.05 | 0.47 ± 0.02 | no data | 0.5d | — |
 | (10h) | 5′-NO2, 6′-OMe | 148 ± 50 | 15 ± 1.6 | no data | 10 | — |
 | (10i) | 5′-I, 6′-OMe | 1.31 ± 0.33 | 2.27 ± 0.31 | 781 ± 181 | 0.6 | 344 |
 | (10j) | 5′-COMe, 6′-OMe | 12.6 ± 3.8 | 15.8 ± 1.65 | 498 ± 24 | 0.8 | 32 |
 | (11a) | 2β-COCH3, 1-naphthyl | 10 ± 2.2 | 26 ± 5.1 | 165 ± 40 | 0.4 | 6.3 |
 | (11b) | 2α-COCH3, 1-naphthyl | 97 ± 21 | 217 ± 55 | no data | 0.45 | — |
 | (11c) | 2α-COCH2CH3, 2-naphthyl | 2.51 ± 0.82 | 16.4 ± 2.0 | 68.0 ± 10.8 | 0.15 | 4.1 |
 | (11d) | 2β-COCH3, 2-naphthyl | 1.27 ± 0.15 | 1.06 ± 0.36 | 4.9 ± 1.2 | 1.2 | 4.6 |
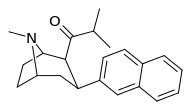 | (11e) | 2β-COCH(CH3)2, 2-naphthyl | 0.25 ± 0.08 | 2.08 ± 0.80 | 37.6 ± 10.5 | 0.12 | 18.1 |
 | (11f) 79a | 2β-COCH2CH3, 2-naphthyl, N8-demethyl | 0.03 ± 0.01 | 0.23 ± 0.07 | 2.05 ± 0.9 | 0.13 | 8.9 |
|
|
Ester reduction
Note: p-fluorophenyl is weaker than the others. RTI-145 is not peroxy, it is a methyl carbonate.

| Code | X | 2 Position | config | 8 | DA | 5-HT | NE |
|---|---|---|---|---|---|---|---|
| RTI-100 | F | -CH2OH | β,β | NMe | 47 | 4741 | no data |
| RTI-101 | I | -CH2OH | β,β | NMe | 2.2 | 26 | no data |
| RTI-99 | Br | -CH2OH | β,β | NMe | 1.49 | 51 | no data |
| RTI-93 | Cl | -CH2OH | β,β | NMe | 1.53 | 204 | 43.8 |
| RTI-105 | Cl | -CH2OAc | β,β | NMe | 1.60 | 143 | 127 |
| RTI-123 | Cl | -CH2OBz | β,β | NMe | 1.78 | 3.53 | 393 |
| RTI-145 | Cl | -CH2OCO2Me | β,β | NMe | 9.60 | 2.93 | 1.48 |
2-Alkane/Alkene
| Structure | Singh's # | R | X | DAT mazindol displacement |
DA uptake | 5-HT Uptake | Selectivity DA uptake/DAT binding |
|---|---|---|---|---|---|---|---|
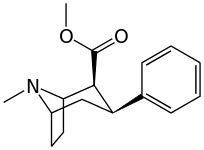 | 11a WIN 35062-2 | 89.4 | 53.7 | 186 | 0.6 | ||
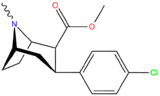 | 11c | 0.83 ± 00.7 | 28.5 ± 0.9 | — | 34.3 | ||
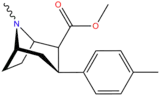 | 11f | 5.76 | 6.92 | 23.2 | 1.2 | ||
 | 41a | (CH2)2CH3 | H | 12.2 | 6.89 | 86.8 | 0.6 |
 | 41b | (CH2)3C6H5 | H | 16 ± 2a | 43 ± 13b | — | 2.7 |
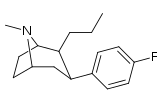 | 42 | (CH2)2CH3 | F | 5.28 | 1.99 | 21.7 | 0.4 |
 | 43a | CH=CH2 | Cl | 0.59 ± 0.15 | 2.47 ± 0.5 | — | 4.2 |
 | 43b | E-CH=CHCl | Cl | 0.42 ± 0.04 | 1.13 ± 0.27 | — | 2.7 |
 | 43c | Z-CH=CHCl | Cl | 0.22 ± 0.02 | 0.88 ± 0.05 | — | 4.0 |
 | 43d | E-CH=CHC6H5 | Cl | 0.31 ± 0.04 | 0.66 ± 0.01 | — | 2.1 |
 | 43e | Z-CH=CHC6H5 | Cl | 0.14 ± 0.07 | 0.31 ± 0.09 | — | 2.2 |
 | 43f | CH2CH3 | Cl | 2.17 ± 0.20 | 2.35 ± 0.52 | — | 1.1 |
 | 43 g | (CH2)2CH3 | Cl | 0.94 ± 0.08 | 1.08 ± 0.05 | — | 1.1 |
 | 43h | (CH2)3CH3 | Cl | 1.21 ± 0.18 | 0.84 ± 0.05 | — | 0.7 |
 | 43i | (CH2)5CH3 | Cl | 156 ± 15 | 271 ± 3 | — | 1.7 |
 | 43j | (CH2)2C6H5 | Cl | 1.43 ± 0.03 | 1.54 ± 0.08 | — | 1.0 |
 | 44a | (CH2)2CH3 | CH3 | 1.57 | 1.10 | 10.3 | 0.7 |
 | 44b | (CH2)3CH3 | CH3 | 1.82 | 1.31 | 15.1 | 0.7 |
 | 45 | (CH2)2CH3 | H | 74.9 | 30.2 | 389 | 0.4 |
 | 46 | (CH2)2CH3 | F | 21.1 | 12.1 | 99.6 | 0.6 |
 | 47a | (CH2)2CH3 | CH3 | 8.91 | 11.8 | 50.1 | 1.3 |
 | 47b | (CH2)3CH3 | CH3 | 11.4 | 10.1 | 51.0 | 0.9 |
aKi value for displacement of WIN 35428.
bIC50 value.


Irreversible covalent (cf. ionic) C2 ligands

Irreversible (phenylisothiocyanate) binding ligand (Murthy, V.; Martin, T. J.; Kim, S.; Davies, H. M. L.; Childers, S. R. (2008). "In Vivo Characterization of a Novel Phenylisothiocyanate Tropane Analog at Monoamine Transporters in Rat Brain". Journal of Pharmacology and Experimental Therapeutics. 326 (2): 587–595. doi:10.1124/jpet.108.138842. PMID 18492949. S2CID 5996473.)[23] RTI-76:[24] 4′-isothiocyanatophenyl (1R,2S,3S,5S)-3-(4-chlorophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate. Also known as: 3β-(p-chlorophenyl)tropan-2β-carboxylic acid p-isothiocyanatophenylmethyl ester.
C2 Acyl, N8 phenylisothiocyanate

HD-205 (Murthy et al., 2007)[25]
Note the contrast to the phenylisothiocyanate covalent binding site locations as compared to the one on p-Isococ, a non-phenyltropane cocaine analogue.
Benztropine based (C2-position hetero-substituted) phenyltropanes


| Structure | Compound | R | X | Y | [3H]WIN 35,428 @ DAT Ki (nM) |
[3H]Citalopram @ SERT Ki (nM) |
[3H]Nisoxetine @ NET Ki (nM) |
[3H]Pirenzepine @ M1 Ki (nM) |
|---|---|---|---|---|---|---|---|---|
 | ||||||||
| 9a | CH3 | H | H | 34 ± 2 | 121 ± 19 | 684 ± 100 | 10,600 ± 1,100 | |
| 9b | F | H | H | 49 ± 12 | — | — | — | |
| 9c | Cl | H | H | 52 ± 2.1 | 147 ± 8 | 1,190 ± 72 | 11,000 ± 1,290 | |
| 9d | CH3 | Cl | H | 80 ± 9 | 443 ± 60 | 4,400 ± 238 | 31,600 ± 4,300 | |
| 9e | F | Cl | H | 112 ± 11 | — | — | — | |
| 9f | Cl | Cl | H | 76 ± 7 | 462 ± 36 | 2,056 ± 236 | 39,900 ± 5,050 | |
| 9g | CH3 | F | F | 62 ± 7 | 233 ± 24 | 1,830 ± 177 | 15,500 ± 1,400 | |
| 9h | F | F | F | 63 ± 13 | — | — | — | |
| 9i | Cl | F | F | 99 ± 18 | 245 ± 16 | 2,890 ± 222 | 16,300 ± 1,300 | |
 | ||||||||
| 10a | CH3 | H | H | 455 ± 36 | 530 ± 72 | 2,609 ± 195 | 12,600 ± 1,790 | |
| 10c | Cl | H | H | 478 ± 72 | 408 ± 16 | 3,998 ± 256 | 11,500 ± 1,720 | |
| 10d | CH3 | Cl | H | 937 ± 84 | 1,001 ± 109 | 22,500 ± 2,821 | 18,200 ± 2,600 | |
| 10f | Cl | Cl | H | 553 ± 106 | 1,293 ± 40 | 5,600 ± 183 | 9,600 ± 600 | |
| 10g | CH3 | F | F | 690 ± 76 | 786 ± 67 | 16,000 ± 637 | 9,700 ± 900 | |
| 10i | Cl | F | F | 250 ± 40 | 724 ± 100 | 52,300 ± 13,600 | 9,930 ± 1,090 | |
 | ||||||||
| 12a | H | H | H | 139 ± 15 | 61 ± 9 | 207 ± 30 | 7,970 ± 631 | |
| 12b | H | Cl | H | 261 ± 19 | 45 ± 3 | — | 24,600 ± 2,930 | |
| 12c | H | F | F | 60 ± 7 | — | — | — |
F&B series (Biotin side-chains etc.)
One patent claims a series of compounds with biotin-related sidechains are pesticides.[18]
| Images of the biotin C2 side-chained phenyltropanes, click to |
|---|
|
|
| Structure | Code | para-X | C2-Tropane Position | config | DA | NE | 5-HT |
|---|---|---|---|---|---|---|---|
.svg.png.webp) | — | H | F1 | β,β | — | — | — |
.svg.png.webp) | RTI-224 | Me | F1c | β,β | 4.49 | — | 155.6 |
 | RTI-233 | Me | F2 | β,β | 4.38 | 516 | 73.6 |
.svg.png.webp) | RTI-235 | Me | F3d | β,β | 1.75 | 402 | 72.4 |
.svg.png.webp) | — | — | F3 | β,β | — | — | — |
 | RTI-236 | Me | B1d | β,β | 1.63 | 86.8 | 138 |
 | RTI-237 | Me | B2d | β,β | 7.27 | 258 | 363 |
 | RTI-244 | Me | B3d | β,β | 15.6 | 1809 | 33.7 |
 | RTI-245 | Cl | F4c | β,β | 77.3 | — | — |
| RTI-246 | Me | F4c | β,β | 50.3 | 3000 | — | |
 | — | — | F5 | β,β | — | — | — |
 | RTI-248 | Cl | F6c | β,β | 9.73 | 4674 | 6.96 |
.svg.png.webp) | RTI-249 | Cl | F1c | β,β | 8.32 | 5023 | 81.6 |
| RTI-266 | Me | F2 | β,β | 4.80 | 836 | 842 | |
| RTI-267 | Me | F7 wrong | β,β | 2.52 | 324 | 455 | |
 | RTI-268 | Me | F7 right | β,β | 3.89 | 1014 | 382 |
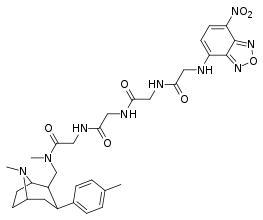 | RTI-269 | Me | F8 | β,β | 5.55 | 788 | 986 |


Miscellany (i.e. Misc./Miscellaneous) C2-substituents




| Structure | Code | X | 2 Position | config | 8 | DA | 5-HT | NE |
|---|---|---|---|---|---|---|---|---|
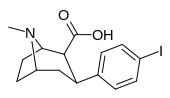 | RTI-102 | I | CO2H | β,β | NMe | 474 | 1928 | 43,400 |
 | RTI-103 | Br | CO2H | β,β | NMe | 278 | 3070 | 17,400 |
 | RTI-104 | F | CO2H | β,β | NMe | 2744 | >100K | >100K |
 | RTI-108 | Cl | -CH2Cl | β,β | NMe | 2.64 | 98 | 129.8 |
 | RTI-241 | Me | -CH2CO2Me | β,β | NMe | 1.02 | 619 | 124 |
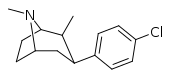 | RTI-139 | Cl | -CH3 | β,β | NMe | 1.67 | 85 | 57 |
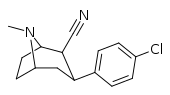 | RTI-161 | Cl | -C≡N | β,β | NMe | 13.1 | 1887 | 2516 |
 | RTI-230 | Cl | H3C–C=CH2 | β,β | NMe | 1.28 | 57 | 141 |
 | RTI-240 | Cl | -CHMe2 | β,β | NMe | 1.38 | 38.4 | 84.5 |
 | RTI-145 | Cl | -CH2OCO2Me | β,β | NMe | 9.60 | 2,932 | 1,478 |
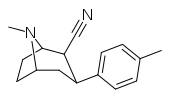 | RTI-158 | Me | -C≡N | β,β | NMe | 57 | 5095 | 1624 |
 | RTI-131 | Me | -CH2NH2 | β,β | NMe | 10.5 | 855 | 120 |
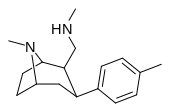 | RTI-164 | Me | -CH2NHMe | β,β | NMe | 13.6 | 2246 | 280 |
 | RTI-132 | Me | -CH2NMe2 | β,β | NMe | 3.48 | 206 | 137 |
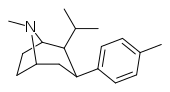 | RTI-239 | Me | -CHMe2 | β,β | NMe | 0.61 | 114 | 35.6 |
 | RTI-338 | Et | -CO2CH2Ph | β,β | NMe | 1104 | 7.41 | 3366 |
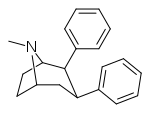 | RTI-348 | H | -Ph | β,β | NMe | 28.2 | >34,000 | 2670 |
C2-truncated/descarboxyl (non-ecgonine w/o 2-position-replacement tropanes)
Aryl-Tropenes
WO 2004113297, Peters, Dan; Olsen, Gunnar M. & Nielsen, Elsebet Oestergaard et al., "Aza-ring derivatives and their use as monoamine neurotransmitter re-uptake inhibitors", published 2004-12-29, assigned to NeuroSearch AS
| Test compound | DA-uptake IC50(μM) | NA-uptake IC50(μM) | 5-HT-uptake IC50(μM) |
|---|---|---|---|
| (+)-3-(4-Chlorophenyl)-8-H-aza-bicyclo[3.2.1]oct-2-ene | 0.26 | 0.028 | 0.010 |
| (+)-3-Napthalen-2-yl-8-azabicyclo[3.2.1]oct-2-ene | 0.058 | 0.013 | 0.00034 |
| (–)-8-Methyl-3-(naphthalen-2-yl)-8-azabicylo[3.2.1]oct-2-ene | 0.034 | 0.018 | 0.00023 |
| Test Compound | DA uptake IC50(μM) | NE uptake IC50(μM) | 5-HT uptake IC50(μM) |
|---|---|---|---|
| (±)-3-(3,4-Dichlorophenyl)-8-methyl-8-azabicyclo[3.2.1]oct-2-ene | 0.079 | 0.026 | 0.0047 |
| Test Compound | DA uptake IC50(μM) | NE uptake IC50(μM) | 5-HT uptake IC50(μM) |
|---|---|---|---|
| (±)-3-(4-cyanophenyl)-8-methyl-8-azabicyclo[3.2.1]oct-2-ene | 18 | 4.9 | 0.047 |
| (±)-3-(4-nitrophenyl)-8-methyl-8-azabicyclo[3.2.1]oct-2-ene | 1.5 | 0.5 | 0.016 |
| (±)-3-(4-trifluoromethoxyphenyl)-8-methyl-8-azabicyclo[3.2.1]oct-2-ene | 22.00 | 8.00 | 0.0036 |
Enantioselective nonstandard configurations (non-2β-,3β-)
β,α Stereochemistry

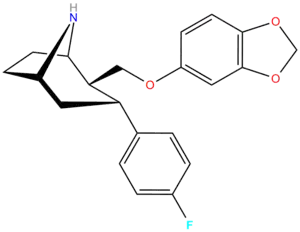
Structure 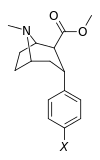 | Compound (RTI #) (S. Singh's #) | X | 2 Group | config | 8 | DAT IC50 (nM) [3H]WIN 35428 | 5-HTT IC50 (nM) [3H]paroxetine | NET IC50 (nM) [3H]nisoxetine | selectivity 5-HTT/DAT | selectivity NET/DAT |
|---|---|---|---|---|---|---|---|---|---|---|
 | RTI-140 20a | H | CO2Me | β,α | NMe | 101 ± 16 | 5,701 ± 721 | 2,076 ± 285 | 56.4 | 20.6 |
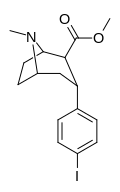 | RTI-352ɑ 20d | I | CO2Me | β,α | NMe | 2.86 ± 0.16 | 64.9 ± 1.97 | 52.4 ± 4.9 | 22.8 | 18.4 |
 | RTI-549 | Br | CO2Me | β,α | NMe | — | — | — | — | — |
 | RTI-319b | 3α-2-naphthyl | CO2Me | β,α | NMe | 1.1 ± 0.09 | 11.4 ± 1.3 | 70.2 ± 6.28 | — | — |
 | RTI-286c 20b | F | CO2Me | β,α | NMe | 21 ± 0.57 | 5062 ± 485 | 1231 ± 91 | 241 | 58.6 |
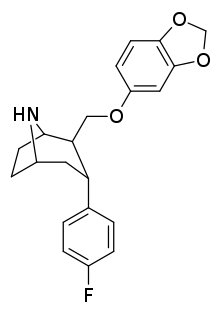 | RTI-274d | F | CH2O(3′,4′-MD-phenyl) | β,α | NH | 3.96 | 5.62 | 14.4 | — | — |
 | RTI-287 | Et | CO2Me | β,α | NMe | 327 | 1687 | 17,819 | — | — |
 | 20c | Cl | CO2Me | β,α | NMe | 2.4 ± 0.2 | 998 ± 120 | 60.1 ± 2.4 | 416 | 25.0 |
 | 20e | Me | CO2Me | β,α | NMe | 10.2 ± 0.08 | 4250 ± 422 | 275 ± 24 | 417 | 27.0 |
 | Bn | CO2Me | β,α | NMe | — | — | — | — | — |

α,β Stereochemistry

| Compound | DA (μM) | M.E.D. (mg/kg) | Dose (mg/kg) | Activity | Activity |
|---|---|---|---|---|---|
| (2R,3S)-2-(4-chlorophenoxymethyl)-8-methyl-3-(3-chlorophenyl)-8-azabicyclo[3.2.1]octane | 0.39 | <1 | 50 | 0 | 0 |
| (2R,3S)-2-(carboxymethyl)-8-methyl-3-(2-naphthyl)-8-azabicyclo[3.2.1]octane | 0.1 | 1 | 25 | 0 | 0 |
| (2R,3S)-2-(carboxymethyl)-8-methyl-3-(3,4-dichlorophenyl)-8-azabicyclo[3.2.1]octane | 0.016 | 0.25 | 50 | + | +++ |
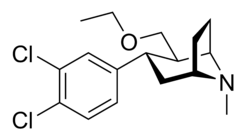

di-chloro; para- & meta- in tandem (α,β configured phenyltropanes)
| Compound | X | 2 Group | config | 8 | DA | 5-HT | NE |
|---|---|---|---|---|---|---|---|
| Brasofensine | Cl2 | methyl aldoxime | α,β | NMe | — | — | — |
| Tesofensine | Cl2 | ethoxymethyl | α,β | NMe | 65 | 11 | 1.7 |
| NS-2359 (GSK-372,475) | Cl2 | Methoxymethyl | α,β | NH | — | — | — |
fumaric acid salts (of α,β configured phenyltropanes)
WO 2004072075, Peters, Dan; Nielsen, Elsebet Oestergaard & Olsen, Gunnar M. et al., "Novel 8-aza-bicyclo[3.2.1]octane derivatives and their use as monoamine neurotransmitter re-uptake inhibitors", published 2004-08-26, assigned to NeuroSearch AS
| Test Compound | DA uptake IC50(μM) | NE uptake IC50(μM) | 5-HT uptake IC50(μM) |
|---|---|---|---|
| (2R,3S)-2-(2,3-dichlorophenoxymethyl)-8-methyl-3-(3-chlorophenyl)-8-azabicyclo[3.2.1]octane fumaric acid salt | 0.062 | 0.035 | 0.00072 |
| (2R,3S)-2-(Naphthaleneoxymethane)-8-methyl-3-(3-chlorophenyl)-8-azabicyclo[3.2.1]octane fumaric acid salt | 0.062 | 0.15 | 0.0063 |
| (2R,3S)-2-(2,3-dichlorophenoxymethyl)-8-H-3-(3-chlorophenyl)-8-azabicyclo[3.2.1]octane fumaric acid salt | 0.10 | 0.048 | 0.0062 |
| (2R,3S)-2-(Naphthlyloxymethane)-8-H-3-(3-chlorophenyl)-8-azabicyclo[3.2.1]octane fumaric acid salt | 0.088 | 0.051 | 0.013 |
Arene equivalent alterations
η6-3β-(transition metal complexed phenyl)tropanes
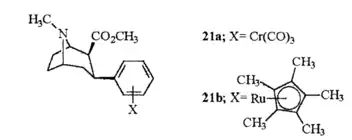
21b can be prepared from ferrocenes and perrhenate by a double ligand transfer (DLT) reaction.[28]
Unlike metal complexed PTs created with the intention of making useful radioligands, 21a & 21b were produced seeing as their η6-coordinated moiety dramatically altered the electronic character and reactivity of the benzene ring, as well as such a change adding asymmetrical molecular volume to the otherwise planar arene ring unit of the molecule.[1] (cf. the Dewar–Chatt–Duncanson model). In addition the planar dimension of the transition metal stacked arene becomes delocalized (cf. Bloom and Wheeler.[29]).
21a was twice as potent as both cocaine and troparil in displacement of β-CFT, as well as displaying high & low affinity Ki values in the same manner as those two compounds. Whereas its inhibition of DA uptake showed it as comparably equipotent to cocaine & troparil. 21b by contrast had a one hundredfold decrease in high-affinity site binding compared to cocaine and a potency 10× less for inhibiting DA uptake. Attesting these as true examples relating useful effective applications for bioorganometallic chemistry.

The discrepancy in binding for the two benzene metal chelates is assumed to be due to electrostatic differences rather than their respective size difference. The solid cone angles, measured by the steric parameter (i.e. θ) is θ=131° for Cr(CO)3 whereas Cp*Ru was θ=187° or only 30% larger. The tricarbonyl moiety being considered equivalent to the cyclopenta dienyl (Cp) ligand.[1]
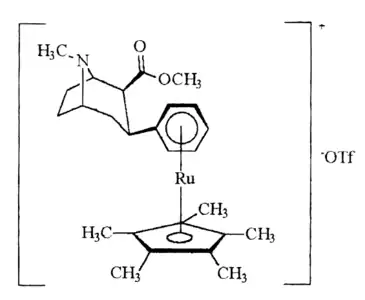
| Structure | Compound # (S. Singh) Systematic name |
Ki (nM)ɑ | IC50 (nM) | selectivity binding/uptake |
|---|---|---|---|---|
 | 21ac | 17 ± 15b 224 ± 83 | 418 | 24.6 |
 | 21bd | 2280 ± 183 | 3890 | 1.7 |
| Cocaine | 32 ± 5 388 ±221 | 405 | 12.6 | |
| Troparil (11a) | 33 ± 17 314 ± 222 | 373 | 11.3 | |
- ɑThe binding data fit a two-site model better than a one-site model
- bThe Ki value for the one-site model was 124 ± 10 nM
- cIUPAC: [η6-(2β-carbomethoxy-3β-phenyl)tropane]tricarbonylchromium
- dIUPAC: [η5-(pentamethylcyclopentadienyl)]-[η6-(2β-carbomethoxy-3β-phenyl)tropane]ruthenium-(II) triflate
3-(2-thiophene) and 3-(2-furan)
| Code | Compound | DA (μM) | NE (μM) | 5-HT (μM) |
|---|---|---|---|---|
| 1 | (2R,3S)-2-(2,3-Dichlorophenoxymethyl)-8-methyl-3-(2-thienyl)-8-aza-bicyclo[3.2.1]octanefumaric acid salt | 0.30 | 0.0019 | 0.00052 |
| 2 | (2R,3S)-2-(1-Naphthyloxymethyl)-8-methyl-3-(2-thienyl)-8-aza-bicyclo-[3.2.1]octane fumaric acid salt | 0.36 | 0.0036 | 0.00042 |
| 3 | (2R,3S)-2-(2,3-Dichlorophenoxymethyl)-8-methyl-3-(2-furanyl)-8-aza-bicyclo-[3.2.1]octane fumaric acid salt | 0.31 | 0.00090 | 0.00036 |
| 4 | (2R,3S)-2-(1-Naphthyloxymethyl)-8-methyl-3-(2-furanyl)-8-aza-bicyclo-[3.2.1]octane fumaric acid salt | 0.92 | 0.0030 | 0.00053 |
| 5 | (2R,3S)-2-(2,3-Dichlorophenoxymethyl)-8-H-3-(2-thienyl)-8-aza-bicyclo[3.2.1]octane fumaric acid salt | 0.074 | 0.0018 | 0.00074 |
| 6 | (2R,3S)-2-(1-Naphthyloxymethyl)-8-H-3-(2-thienyl)-8-aza-bicyclo[3.2.1]octane fumaric acid salt | 0.19 | 0.0016 | 0.00054 |
Thiophenyltropanes

Diaryl
 ZIENT:[32] |
6/7-tropane position substituted
2β-carbomethoxy 6/7 substituted
| Structure | Compound # (S. Singh) |
Substitution | DAT (IC50 nM) displacement of [H3]WIN 35428 |
5-HTT (IC50 nM) [H3]Citalopram |
Selectivity 5-HTT/DAT |
|---|---|---|---|---|---|
| Cocaine | H | 65 ± 12 | - | - | |
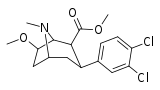 | 103a | 3β,2β, 7-OMe 3′,4′-Cl2 | 86 ± 4.7 | 884 ± 100 | 10.3 |
 | 103b | 3β,2β, 7-OH 3′,4′-Cl2 | 1.42 ± 0.03 | 28.6 ± 7.8 | 20.1 |
 | 103c | 3α,2β, 7-OH 3′,4′-Cl2 | 1.19 ± 0.16 | 1390 ± 56 | 1168 |
 | 104a | 3β,2β, 6-OH 4′-Me | 215ɑ | - | - |
 | 104b | 3β,2α, 6-OH 4′-Me | 15310ɑ | - | - |
 | 104c | 3α,2β, 6-OH 4′-Me | 930ɑ | - | - |
 | 104d | 3α,2α, 6-OH 4′-Me | 7860ɑ | - | - |
- ɑIC50 value for displacement of [H3]mazindol. IC50 for cocaine 288 nM for displacement of [H3]mazindol
3-butyl 6/7 substituted
| Structure | Compound # (S. Singh) |
Substituent | Ki nM displacement of [H3]mazindol binding |
Ki nM [H3]DA uptake |
Selectivity uptake/binding |
|---|---|---|---|---|---|
| Cocaine | H | 270 ± 0.03 | 400 ± 20 | 1.5 | |
 | 121a | 7β-CN | 2020 ± 10 | 710 ± 40 | 0.3 |
 | 121b | 6β-CN | 3040 ± 480 | 6030 ± 880 | 2.0 |
 | 121c | 7β-SO2Ph | 4010 ± 310 | 8280 ± 1340 | 2.1 |
 | 121d | 6β-SO2Ph | 4450 ± 430 | 8270 ± 690 | 1.8 |
 | 121e | 7α-OH | 830 ± 40 | 780 ± 60 | 0.9 |
 | 121f | H | 100 ± 10 | 61 ± 10 | 0.6 |
 | 121g | 7β-CN | 24000 ± 3420 | 32100 ± 8540 | 1.3 |
 | 121h | 6β-CN | 11300 ± 1540 | 26600 ± 3330 | 2.3 |
 | 121i | 7β-SO2Ph | 7690 ± 2770 | 7050 ± 450 | 0.9 |
 | 121j | 6β-SO2Ph | 4190 ± 700 | 8590 ± 1360 | 2.0 |
 | 121k | 7α-SO2Ph | 3420 ± 1100 | - | - |
 | 121l | 7β-SO2Ph, 7α-F | 840 ± 260 | 2520 ± 290 | 3.0 |
 | 121m | 7α-F | 200 ± 10 | 680 ± 10 | 3.4 |
 | 121n | 7β-F | 500 ± 10 | 550 ± 140 | 1.1 |
intermediate 6- & 7-position synthesis modified phenyltropanes
| Structure | Compound # (S. Singh) |
Substituent W | Substituent X | Substituent Y | Substituent Z |
|---|---|---|---|---|---|
 | (±)-122a | CN | H | H | H |
 | (±)-122b | H | H | CH | H |
 | (±)-122c | H | CH | H | H |
 | (±)-122d | H | H | H | CH |
 | (±)-122e | SO2Ph | H | H | H |
 | (±)-122f | H | H | SO2Ph | H |
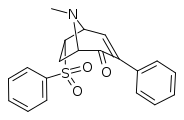 | (±)-122g | H | SO2Ph | H | H |
 | (±)-122h | SO2Ph | F | H | H |
 | (±)-122i | F | SO2Ph | H | H |
 | (±)-122j | H | H | SO2Ph | F |
8-tropane (bridgehead) position modified
Nortropanes (N-demethylated)

NS2359 (GSK-372,475)
It is well established that electrostatic potential around the para position tends to improve MAT binding. This is believed to also be the case for the meta position, although it is less studied. N-demethylation dramatically potentiates NET and SERT affinity, but the effects of this on DAT binding are insignificant.[33] Of course, this is not always the case. For an interesting exception to this trend, see the Taxil document. There is ample evidence suggesting that N-demethylation of alkaloids occurs naturally in vivo via a biological enzyme. The fact that hydrolysis of the ester leads to inactive metabolites means that this is still the main mode of deactivation for analogues that have an easily metabolised 2-ester substituent. The attached table provides good illustration of the effect of this chemical transformation on MAT binding affinities. N.B. In the case of both nocaine and pethidine, N-demethyl compounds are more toxic and have a decreased seizure threshold.[34]
| Code (S.S. #) |
X para (unless position otherwise given inline) |
DA | 5HT | NE |
|---|---|---|---|---|
| RTI-142 75b | F | 4.39 | 68.6 | 18.8 |
| RTI-98 75d Norɑ-RTI-55 | I | 0.69 | 0.36 | 11.0 |
| RTI-110 75c | Cl | 0.62 | 4.13 | 5.45 |
| RTI-173 75f | Et | 49.9 | 8.13 | 122 |
| RTI-279 Norɑ-RTI-280 | para-Me meta-I | 5.98 ± 0.48 | 1.06 ± 0.10 | 74.3 ± 3.8 |
| RTI-305 Norɑ-RTI-360/11y | Ethynyl | 1.24 ± 0.11 | 1.59 ± 0.2 | 21.8 ± 1.0 |
| RTI-307 Norɑ-RTI-281/11z | Propynyl | 6.11 ± 0.67 | 3.16 ± 0.33 | 115.6 ± 5.1 |
| RTI-309 Norɑ-11t | Vinyl | 1.73 ± 0.05 | 2.25 ± 0.17 | 14.9 ± 1.18 |
| RTI-330 Norɑ-11s | Isopropyl | 310.2 ± 21 | 15.1 ± 0.97 | — |
| RTI-353 | para-Et meta-I | 330.54 ± 17.12 | 0.69 ± 0.07 | 148.4 ± 9.15 |
ɑThe N-demethylated variant of (i.e. compound code-name after dash)
| N-Me compound code# → N-demethylated derivative compound code # |
para-X | [3H]Paroxetine | [3H]WIN 35,428 | [3H]Nisoxetine |
|---|---|---|---|---|
| 11 g→75f | Ethyl | 28.4 → 8.13 | 55 → 49.9 | 4,029 → 122 |
| 11t→75i | Vinyl | 9.5 → 2.25 | 1.24 → 1.73 | 78 → 14.9 |
| 11y→75n | Ethynyl | 4.4 → 1.59 | 1.2 → 1.24 | 83.2 → 21.8 |
| 11r→75 g | 1-Propyl | 70.4 → 26 | 68.5 → 212 | 3,920 → 532 |
| 11v→75k | trans-propenyl | 11.4 → 1.3 | 5.29 → 28.6 | 1,590 → 54 |
| 11w→75l | cis-propenyl | 7.09 → 1.15 | 15 → 31.6 | 2,800 → 147 |
| 11x→75 m | Allyl | 28.4 → 6.2 | 32.8 → 56.5 | 2,480 → 89.7 |
| 11z→75o | 1-Propynyl | 15.7 → 3.16 | 2.37 → 6.11 | 820 → 116 |
| 11s→75h | i-Propyl | 191 → 15.1 | 597 → 310 | 75,000 → ? |
| 11u→75j | 2-Propenyl | 3.13 → 0.6 | 14.4 → 23 | 1,330? → 144 |
| Isomer | 4′ | 3′ | NE | DA | 5HT |
|---|---|---|---|---|---|
| β,β | Me | H | 60 → 7.2 | 1.7 → 0.84 | 240 → 135 |
| β,β | F | H | 835 → 18.8 | 15.7 → 4.4 | 760 → 68.6 |
| β,β | Cl | H | 37 → 5.45 | 1.12 → 0.62 | 45 → 4.13 |
| β,α | Me | H | 270 → 9 | 10.2 → 33.6 | 4250 → 500 |
| β,α | F | H | 1200 → 9.8 | 21 → 32.6 | 5060 → 92.4 |
| β,α | Cl | H | 60 → 5.41 | 2.4 → 3.1 | 998 → 53.3 |
| β,α | F | Me | 148 → 4.23 | 13.7 → 9.38 | 1161 → 69.8 |
| β,α | Me | F | 44.7 → 0.86 | 7.38 → 9 | 1150 → 97.4 |
"Interest in NET selective drugs continues as evidenced by the development of atomoxetine, manifaxine, and reboxetine as new NET selective compounds for treating ADHD and other CNS disorders such as depression" (FIC, et al. 2005).[35]
| Structure | Short Name (S. Singh) |
Para-X | DAT [3H]WIN 35428 IC50 (nM) |
5-HTT [3H]Paroxetine IC50 (nM) |
NET [3H]Nisoxetine IC50 (nM) |
Selectivity 5-HTT/DAT |
Selectivity NET/DAT |
|---|---|---|---|---|---|---|---|
| Norcocaine | H | 206 ± 29 | 127 ± 13 | 139 ± 9 | 0.6 | 0.7 | |
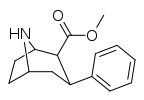 | 75a | H | 30.8 ± 2.3 | 156 ± 8 | 84.5 ± 7.5 | 5.1 | 2.7 |
 | 75b | F | 4.39 ± 0.20 | 68.6 ± 2.0 | 18.8 ± 0.7 | 15.6 | 4.3 |
 | 75c | Cl | 0.62 ± 0.09 | 4.13 ± 0.62 | 5.45 ± 0.21 | 6.7 | 8.8 |
 | 75d | I | 0.69 ± 0.2 | 0.36 ± 0.05 | 7.54 ± 3.19 | 0.5 | 10.9 |
 | 75e | para-I & 2β-CO2CH(CH3)2 | 1.06 ± 0.12 | 3.59 ± 0.27 | 132 ± 5 | 3.4 | 124 |
 | 75f | C2H5 | 49.9 ± 7.3 | 8.13 ± 0.30 | 122 ± 12 | 0.2 | 2.4 |
 | 75g | n-C3H7 | 212 ± 17 | 26 ± 1.3 | 532 ± 8.1 | 0.1 | 2.5 |
 | 75h | CH(CH3)2 | 310 ± 21 | 15.1 ± 0.97 | - | 0.05 | - |
 | 75i | CH=CH2 | 1.73 ± 0.05 | 2.25 ± 0.17 | 14.9 ± 1.18 | 1.3 | 8.6 |
 | 75j | C-CH3 ║ CH2 | 23 ± 0.9 | 0.6 ± 0.06 | 144 ± 12 | 0.03 | 6.3 |
 | 75k | trans-CH=CHCH3 | 28.6 ± 3.1 | 1.3 ± 0.1 | 54 ± 16 | 0.04 | 1.9 |
 | 75l | cis-CH=CHCH3 | 31.6 ± 2.2 | 1.15 ± 0.1 | 147 ± 4.3 | 0.04 | 4.6 |
 | 75m | CH2CH=CH2 | 56.5 ± 56 | 6.2 ± 0.3 | 89.7 ± 9.6 | 0.1 | 1.6 |
 | 75n | CH≡CH | 1.24 ± 0.11 | 1.59 ± 0.2 | 21.8 ± 1.0 | 1.3 | 17.6 |
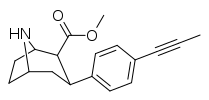 | 75o | CH≡CCH3 | 6.11 ± 0.67 | 3.16 ± 0.33 | 116 ± 5.1 | 0.5 | 19.0 |
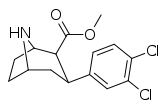 | 75pɑ | 3,4-Cl2 | 0.66 ± 0.24 | 1.4b | - | 2.1 | - |
ɑThese values determined in Cynomolgus monkey caudate-putamen bThe radioligand used for 5-HTT was [3H]citalopram
| Compound Structure | Short Name (S. Singh) |
DAT [125I]RTI-55 IC50 (nM) |
5-HTT [3H]Paroxetine Ki (nM) |
NET [3H]Nisoxetine Ki (nM) |
Selectivity 5-HTT/DAT |
Selectivity NET/DAT |
|---|---|---|---|---|---|---|
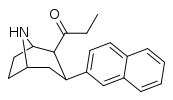 | 79a | 0.07 ± 0.01 | 0.22 ± 0.16 | 2.0 ± 0.09 | 3.1 | 28.6 |
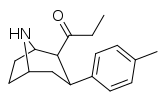 | 79b | 4.7 ± 0.58 | 19 ± 1.4 | 5.5 ± 2.0 | 4.0 | 1.2 |
 | 79c | 380 ± 110 | 5.3 ± 1.0 | 3400 ± 270 | 0.01 | 8.9 |
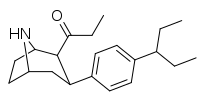 | 79d | 190 ± 17 | 150 ± 50 | 5100 ± 220 | 0.8 | 26.8 |
 | 79e | 490 ± 120 | 85 ± 16 | 4300 ± 1100 | 0.1 | 8.8 |
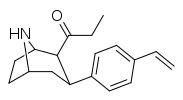 | 79f | 1.5 ± 1.1 | 0.32 ± 0.06 | 10.9 ± 1.5 | 0.2 | 7.3 |
 | 79g | 16 ± 4.9 | 0.11 ± 0.02 | 94 ± 18 | 0.07 | 5.9 |
Paroxetine homologues
See the N-methyl paroxetine homologues cf. di-aryl phenyltropanes for another SSRI approximated hybrid: the fluoxetine based homologue of the phenyltropane class.
| Compound Structure | Short Name (S. Singh) |
Stereochemistry | DAT [3H]WIN 35428 IC50 (nM) |
5-HTT [3H]Paroxetine IC50 (nM) |
NET [3H]Nisoxetine IC50 (nM) |
Selectivity 5-HTT/DAT |
Selectivity NET/DAT |
|---|---|---|---|---|---|---|---|
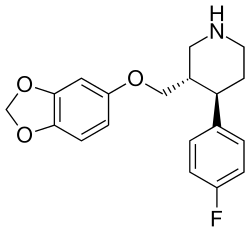 | Paroxetine | - | 623 ± 25 | 0.28 ± 0.02 | 535 ± 15 | 0.0004 | 0.8 |
 | R-81a | 2β,3β | 835 ± 90 | 480 ± 21 | 37400 ± 1400 | 0.6 | 44.8 |
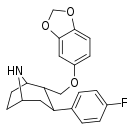 | R-81b | 2α,3β | 142 ± 13 | 90 ± 3.4 | 2500 ± 250 | 0.6 | 17.6 |
 | R-81c | 2β,3α | 3.86 ± 0.2 | 5.62 ± 0.2 | 14.4 ± 1.3 | 1.4 | 3.7 |
 | S-81d | 2β,3β | 1210 ± 33 | 424 ± 15 | 17300 ± 1800 | 0.3 | 14.3 |
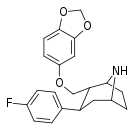 | S-81e | 2α,3β | 27.6 ± 2.4 | 55.8 ± 5.73 | 1690 ± 150 | 2.0 | 61.2 |
 | S-81f | 2β,3α | 407 ± 33 | 19 ± 1.8 | 1990 ± 176 | 0.05 | 4.9 |
N-replaced (S,O,C)


The eight position nitrogen has been found to not be an exclusively necessary functional anchor for binding at the MAT for phenyltropanes and related compounds. Sulfurs, oxygens, and even the removal of any heteroatom, leaving only the carbon skeleton of the structure at the bridged position, still show distinct affinity for the monoamine transporter cocaine-target site and continue to form an ionic bond with a measurable degree of reasonable efficacy.


| Compound | X | 2 Group | config | 8 | DA | 5-HT | NE |
| Tropoxane | Cl,Cl | CO2Me | (racemic) β,β | O | 3.3 | 6.5 | No data |
| O-4210[36] | p-F | 3-methyl-5-isoxazole | β,β | S | 7.0 | >1000 | No data |
-8-methyl-8-azabicyclo(3.2.1)oct-2-ene-2-carbonyl)-3%CE%BB%E2%81%B6-thia-4-azatricyclo(5.2.1.0%C2%B9%252C%E2%81%B5)decane-3%252C3-dione.svg.png.webp)
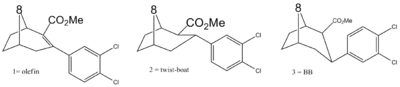
8-oxa bridgehead replacements
| Structure | Compound # (S. Singh) |
Para- (meta-) |
DAT (IC50 nM) displacement of [H3]WIN 35428 |
5-HTT (IC50 nM) [H3]Citalopram |
Selectivity 5-HTT/DAT |
|---|---|---|---|---|---|
 | R/S-90a | H | >1000 | >1000 | - |
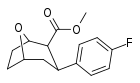 | R/S-90b | F | 546 | 2580 | 4.7 |
 | R/S-90c | Cl | 10 | 107 | 10.7 |
 | R/S-90d | Br | 22 | 30 | 1.4 |
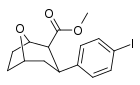 | R/S-90e | I | 7 | 12 | 1.7 |
 | R/S-90f | 3,4-Cl2 | 3.35 | 6.52 | 1.9 |
 | R-90g | 3,4-Cl2 | 3.27 | 4.67 | 1.4 |
 | S-90h | 3,4-Cl2 | 47 | 58 | 1.2 |
 | R/S-91a | H | 1990 | 11440 | 5.7 |
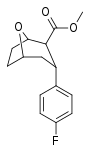 | R/S-91b | F | >1000 | >10000 | - |
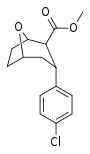 | R/S-91c | Cl | 28.5 | 816 | 28.6 |
 | R/S-91d | Br | 9 | 276 | 30.7 |
 | R/S-91e | I | 42 | 72 | 1.7 |
 | R/S-91f | 3,4-Cl2 | 3.08 | 64.5 | 20.9 |
 | R-91g | 3,4-Cl2 | 2.34 | 31 | 13.2 |
 | S-91h | 3,4-Cl2 | 56 | 2860 | 51.1 |
8-carba bridgehead replacements
| Structure | Compound # (S. Singh) |
DAT (IC50 nM) displacement of [H3]WIN 35428 |
5-HTT (IC50 nM) [H3]Citalopram |
Selectivity 5-HTT/DAT |
|---|---|---|---|---|
 | R/S-98a | 7.1 ± 1.7 | 5160 ± 580 | 726 |
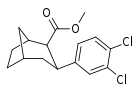 | R/S-98b | 9.6 ± 1.8 | 33.4 ± 0.6 | 3.5 |
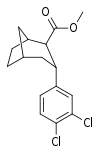 | R/S-98c | 14.3 ± 1.1 | 180 ± 65 | 12.6 |
N-alkyl



| Compound | X | 2 Group | config | 8 | DAT | SERT | NET |
|---|---|---|---|---|---|---|---|
| FP-β-CPPIT | Cl | 3′-phenylisoxazol-5′-yl | β,β | NCH2CH2CH2F | - | - | - |
| FE-β-CPPIT | Cl | (3′-phenylisoxazol-5′-yl) | β,β | NCH2CH2F | - | - | - |
| Altropane (IACFT) | F | CO2Me | β,β | NCH2CH=CHF | - | - | - |
| FECNT[37] | I | CO2Me | β,β | NCH2CH2F | - | - | - |
| RTI-310 U.S. Patent 5,736,123 | I | CO2Me | β,β | N-Prn | 1.17 | - | - |
| RTI-311 | I | CO2Me | β,β | NCH2CH=CH2 | 1.79 | - | - |
| RTI-312 U.S. Patent 5,736,123 | I | CO2Me | β,β | NBun | 0.76 | - | - |
| RTI-313 U.S. Patent 5,736,123 | I | CO2Me | β,β | NCH2CH2CH2F | 1.67 | - | - |
| Ioflupane (FP-CIT) | 123I | CO2Me | β,β | NCH2CH2CH2F | - | - | - |
| PE2I[37] | Me | CO2Me | β,β | NCH2CH=CHI | - | - | - |
| RTI-251 | Cl | CO2Me | β,β | NCH2CO2Et | 1.93 | 10.1 | 114 |
| RTI-252 | Cl | CO2Me | β,β | NCH2CH2CO2Et | 2.56 | 35.2 | 125 |
| RTI-242 | Cl | β,β (bridged) -C(O)CH(CO2Me)CH2N | 7.67 | 227 | 510 | ||
Bi- and tri-cyclic aza compounds and their uses.[38][39]
| Structure | Short Name (S. Singh) |
Nitrogen side-chain (N8) |
DAT [3H]GBR 12935 Ki (nM) |
5-HTT [3H]Paroxetine Ki (nM) |
NET [3H]Nisoxetine Ki (nM) |
Selectivity 5-HTT/DAT |
Selectivity NET/DAT |
|---|---|---|---|---|---|---|---|
| Cocaine | H | 350 ± 80 | >10000 | >30000 | >28.6 | - | |
| GBR 12909 | - | 0.06 ± 0.02 | 52.8 ± 4.4 | >20000 | 880 | - | |
| WIN 35428 11b | H | 14.7 ± 2.9 | 181 ± 21 | 635 ± 110 | 12.3 | 43.2 | |
| RTI-55 11e | H | 1.40 ± 0.20 | 0.46 ± 0.06 | 2.80 ± 0.40 | 0.3 | 2 | |
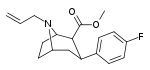 | 82a | CH2CH=CH2 | 22.6 ± 2.9ɑ | - | - | - | - |
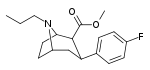 | 82b | CH2CH2CH3 | 43.0 ± 17.7ɑ | - | - | - | - |
 | 82c | CH2C6H5 | 58.9 ± 1.65b | 1073c | - | 18.2 | - |
 | 82d | (CH2)3C6H5 | 1.4 ± 0.2b | 133 ± 7c | - | 95.0 | - |
 | 82e | (CH2)5C6H5 | 3.4 ± 0.83b | 49.9 ± 10.2c | - | 14.7 | - |
 | 83a | CH2CH2CH2F | 1.20 ± 0.29 | 48.7 ± 8.4 | 10000 | 40.6 | 8333 |
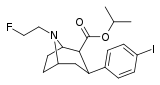 | 83b | CH2CH2F | 4.40 ± 0.35 | 21.7 ± 8.3 | >10000 | 4.9 | - |
 | 84a | CH2CH2CH2F | 3.50 ± 0.39 | 0.110 ± 0.02 | 63.0 ± 4.0 | 0.03 | 18 |
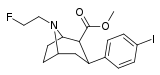 | 84b | CH2CH2F | 4.00 ± 0.73 | 0.140 ± 0.02 | 93.0 ± 17.0 | 0.03 | 23.2 |
 | 84c | CH2CHF2 | 15.1 ± 3.7 | 9.6 ± 1.5 | >5000 | 0.6 | - |
 | 84d | CH2CH2CH2Cl | 3.10 ± 0.57 | 0.32 ± 0.06 | 96.0 ± 29.0 | 0.1 | 31.0 |
 | 84e | CH2CH2CH2Br | 2.56 ± 0.57 | 0.35 ± 0.08 | 164 ± 47 | 0.1 | 64.1 |
 | 84f | CH2CH2CH2I | 38.9 ± 6.3 | 8.84 ± 0.53 | 5000 | 0.2 | 128 |
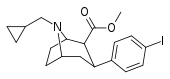 | 84g | CH2...methylcyclopropane | 4.30 ± 0.87 | 1.30 ± 0.25 | 198 ± 9.6 | 0.3 | 46.0 |
 | 84h | CH2CH2CH2OH | 5.39 ± 0.21 | 2.50 ± 0.20 | 217 ± 19 | 0.5 | 40.2 |
 | 84i | CH2CH2(OCH3)2 | 6.80 ± 1.10 | 1.69 ± 0.09 | 110 ± 7.7 | 0.2 | 16.2 |
 | 84j | CH2CO2CH3 | 11.9 ± 1.4 | 0.81 ± 0.10 | 29.1 ± 1.0 | 0.07 | 2.4 |
 | 84k | CH2CON(CH3)2 | 12.2 ± 3.8 | 6.40 ± 1.70 | 522 ± 145 | 0.5 | 42.8 |
 | 84l | CH2CH2CH2OMs | 36.3 ± 2.1 | 17.3 ± 1.2 | 5000 | 0.5 | 138 |
 | 84m | COCH(CH3)2 | 2100 ± 140 | 102 ± 23 | >10000 | 0.05 | - |
 | 84n | (CH2)2Pht | 4.23 ± 0.48 | 0.84 ± 0.02 | 441 ± 66.0 | 0.2 | 104 |
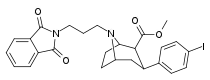 | 84o | (CH2)3Pht | 9.10 ± 1.10 | 0.59 ± 0.07 | 74.0 ± 11.6 | 0.06 | 8.1 |
 | 84p | (CH2)4Pht | 2.38 ± 0.22 | 0.21 ± 0.02 | 190 ± 18.0 | 0.09 | 79.8 |
 | 84q | (CH2)5Pht | 2.40 ± 0.17 | 0.34 ± 0.03 | 60.0 ± 3.10 | 0.1 | 25.0 |
 | 84r | (CH2)8Pht | 2.98 ± 0.30 | 0.20 ± 0.02 | 75.0 ± 3.6 | 0.07 | 25.2 |
 | 84sd | CH2CH=CH-CH3 | 15 ± 1 | 75 ± 5 | 400 ± 80 | 5.0 | 26.7 |
 | 84td | CH2C(Br)=CH2 | 30 ± 5 | 200 ± 40 | >1000 | 6.7 | - |
 | 84ud | CH2CH=CH2I(E) | 30 ± 5 | 960 ± 60 | 295 ± 33 | 32.0 | 9.8 |
 | 84vd | CH2C≡CH | 14 ± 1 | 100 ± 30 | >1000 | 7.1 | - |
 | 84wd | CH2C6H5 | 42 ± 12 | 100 ± 17 | 600 ± 100 | 2.4 | 14.3 |
 | 84xd | CH2C6H4-2-CH3 | 93 ± 19 | 225 ± 40 | >1000 | 2.4 | - |
 | 85ad | para-H | 113 ± 41 | 100 ± 20 | >1000 | 0.9 | - |
 | 85bd | para-Cl, meta-Cl | 29 ± 4 | 50 ± 6 | 500 ± 120 | 1.7 | 17.2 |
 | 85cd | para-Me | 17 ± 7 | 500 ± 30 | >1000 | 29.4 | - |
 | 85dd | para-CH(CH3)2 | 500 ± 120 | 450 ± 80 | >1000 | 0.9 | - |
 | 85ed | para-n-C3H7 | 500 ± 100 | 300 ± 12 | 750 ± 160 | 0.6 | 1.5 |
- ɑIC50 for displacement of [3H]cocaine. IC50 for cocaine = 67.8 ± 8.7 (nM)
- bIC50 values for displacement of [3H]WIN 35428
- cIC50 values for displacement of [3H]citalopram
- dThe standard Ki value for the displacement of [3H]GBR 12935, [3H]paroxetine, and [3H]nisoxetine were 27 ± 2, 3 ± 0.2, and 80 ± 28 nM, respectively, for these experiments
Structure  |
Compound | R1 | R2 | Inhibition of [3H]WIN 35,428 @ DAT IC50 (nM) |
Inhibition of [3H]Paroxetine @ 5-HTT Ki (nM) |
Inhibition of [3H]Nisoxetine @ NET Ki (nM) |
NET/DAT (uptake ratio) |
NET/5-HTT (uptake ratio) |
|---|---|---|---|---|---|---|---|---|
| See 7a—7h table | ||||||||
| 7a | CH3 | CH3 | 9 ± 3 | 0.7 ± 0.2 | 220 ± 10 | 24 | 314 | |
| 7b | C2H5 | CH3 | 232 ± 34 | 4.5 ± 0.5 | 1170 ± 300 | 5 | 260 | |
 | 8a | CH3 | H | 28 ± 6 | 0.19 ± 0.01 | 21 ± 6 | 0.8 | 110 |
 | 8b | C2H5 | H | 177 ± 62 | 1.26 ± 0.05 | 118 ± 13 | 0.7 | 94 |
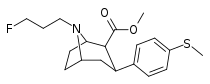 | 9a | CH3 | FCH2CH2CH2 | 112 ± 2 | 3 ± 1 | 960 ± 100 | 9 | 320 |
 | 9b | C2H5 | FCH2CH2CH2 | 1,200 ± 200 | 27 ± 2 | >2,000 | 2 | 74 |
 | 10a | CH3 | CH2=CH2CH2 | 71 ± 25 | 5.5 ± 0.8 | 2,000 ± 500 | 28 | 364 |
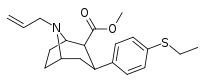 | 10b | C2H5 | CH2=CH2CH2 | 1,100 ± 100 | 47 ± 3 | >2,000 | 2 | 43 |
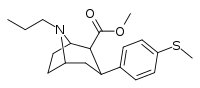 | 11a | CH3 | CH3CH2CH2 | 74 ± 20 | 5.7 ± 0.6 | 1,200 ± 140 | 16 | 211 |
 | 11b | C2H5 | CH3CH2CH2 | 900 ± 300 | 49 ± 6 | >2,000 | 2 | 41 |
Bridged N-constrained phenyltropanes (fused/tethered)
p-methyl aryl front & back N-bridged phenyltropanes

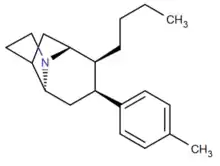
| Compound # (S. Singh's #) |
2β=R | [3H]Mazindol binding | [3H]DA uptake | [3H]5-HT uptake | [3H]NE uptake | selectivity [3H]5-HT/[3H]DA |
|---|---|---|---|---|---|---|
| cocaine | CO2CH3 | 375 ± 68 | 423 ± 147 | 155 ± 40 | 83.3 ± 1.5 | 0.4 |
| (–)-40 (–)-128 | 54.3 ± 10.2 | 60.3 ± 0.4 | 1.76 ± 0.23 | 5.24 ± 0.07 | 0.03 | |
| (+)-40 (+)-128 | 79 ± 19 | 114 ± 28 | 1.48 ± 0.07 | 4.62 ± 0.31 | 0.01 | |
| (±)-40 (±)-128 | 61.7 ± 8.5 | 60.3 ± 0.4 | 2.32 ± 0.23 | 2.69 ± 0.12 | 0.04 | |
| 29β | 620 | 1420 | 8030 | — | — | |
| 30β | 186 | 492 | 97.7 | — | — | |
| 31β | 47.0 | 211 | 28.5 | — | — | |
| 29α | 4140 | 20100 | 3920 | — | — | |
| 30α | 3960 | 8850 | 696 | 1150 | — | |
| 45 129 | 6.86 ± 0.43 | 24.0 ± 1.3 | 1.77 ± 0.04 | 1.06 ± 0.03 | 0.07 | |
| 42a 131a | n-Bu | 4.00 ± 0.07 | 2.23 ± 0.12 | 14.0 ± 0.6 | 2.99 ± 0.17 | 6.3 |
| 41a 130a | n-Bu | 17.2 ± 1.13 | 10.2 ± 1.4 | 78.9 ± 0.9 | 15.0 ± 0.4 | 7.8 |
| 42b 131b | Et | 3.61 ± 0.43 | 11.3 ± 1.1 | 25.7 ± 4.3 | 4.43 ± 0.01 | 2.3 |
| 50a 133a | n-Bu | 149 ± 6 | 149 ± 2 | 810 ± 80 | 51.7 ± 12 | 5.4 |
| 49a 132a | n-Bu | 13.7 ± 0.8 | 14.2 ± 0.1 | 618 ± 87 | 3.84 ± 0.35 | 43.5 |
| (–)-4 | 10500 | 16500 | 1890 | 70900 | — | |
| (+)-4 | 18500 | 27600 | 4630 | 38300 | — | |
| (–)-5 | 9740 | 9050 | 11900 | 4650 | — | |
| (+)-5 | 6770 | 10500 | 25100 | 4530 | — | |
| RTI-4229/Coc-242 | N8/2β-C(O)CH(CO2Me)CH2N para-chloro | — | 7.67 ± 0.31ɑ | 226.54 ± 27.37b | 510.1 ± 51.4c | — |
- ɑValue for displacement of [3H]WIN 35,428 binding @ DAT
- bValue for displacement of [3H]paroxetine binding to SERT
- cValue for displacement of [3H]nisoxetine from NET
Fused tropane-derivatives as neurotransmitter reuptake inhibitors. Singh notes that all bridged derivatives tested displayed 2.5—104 fold higher DAT affinity than cocaine. The ones 2.8—190 fold more potent at DAT also had increased potency at the other two MAT sites (NET & SERT); NET having 1.6—78× increased activity. (+)-128 additionally exhibited 100× greater potency @ SERT, whereas 132a & 133a had 4—5.2× weaker 5-HTT (i.e. SERT) activity. Front-bridged (e.g. 128 & 129) had a better 5-HT/DA reuptake ratio in favor of SERT, while the back-bridged (e.g. 130—133) preferred placement with DAT interaction.[1] U.S. Patent 5,998,405
3,4-Cl2 aryl front-bridged phenyltropanes


| Code | Compound | DA (μM) | NE (μM) | 5-HT (μM) |
|---|---|---|---|---|
| 1 | (1 S,2S,4S,7R)-2-(3,4-Dichloro- phenyl)-8-azatricyclo[5.4.0.04,8]- undecan-11 -one O-methyl-oxime | 0.012 | 0.0020 | 0.0033 |
| 2 | (1 S,2S,4S,7R)-2-(3,4-Dichloro- phenyl)-8-azatricyclo[5.4.0.04,8]- undecan-11-one | 0.18 | 0.035 | 0.0075 |
| 3 | (1 S,3S,4S,8R)-3-(3,4-Dichloro-phenyl)-7-azatricyclo[5.3.0.04,8]- decan-5-one O-methyl-oxime | 0.0160 | 0.0009 | 0.0032 |
| 4 | (1 S,2S,4S,7R)-2-(3,4-Dichloro-phenyl)-8-azatricyclo[5.4.0.04,8]- undecan-11-ol | 0.0750 | 0.0041 | 0.0028 |
| 5 | (1 S,3S,4S,8R)-3-(3,4-Dichloro-phenyl)-7-azatricyclo[5.3.0.04,8]- decan-5-one | 0.12 | 0.0052 | 0.0026 |
| 6 | (1 S,3S,4S,8R)-3-(3,4-Dichloro- phenyl)-7-azatricyclo[5.3.0.04,8]-decan-5-ol | 0.25 | 0.0074 | 0.0018 |
| 7 | (1S,3S,4S,8R)-3- (3,4-Dichloro- phenyl)-7-azatricyclo[5.3.0.04,8]dec- 5-yl acetate | 0.21 | 0.0061 | 0.0075 |
| 8 | (1S,3S,4S,8R)-3-(3,4-Dichlorophenyl)-5-methoxy-7- azatricyclo[5.3.0.04,8]decane | 0.022 | 0.0014 | 0.0001 |
- 1-Chloroethyl chloroformate is used to remove N-methyl of trans-aryltropanes.
- 2° amine is reacted with Br(CH2)nCO2Et.
- Base used to abstract proton α- to CO2Et group and complete the tricyclic ring closure step (Dieckmann cyclization).
To make a different type of analog (see Kozikowski patent above)
- Remove N-Me
- Add ɣ-bromo-chloropropane
- Allow for cyclization with K2CO3 base and KI cat.
C2 + C3 (side-chain) fused (carboxylate & benzene conjoined)
-3-(4-methylphenyl)-9%252C18-diazapentacyclo(9.7.0.0%C2%B2%252C%E2%81%B8.0%E2%81%B5%252C%E2%81%B9.0%C2%B9%C2%B2%252C%C2%B9%E2%81%B7)octadeca-1(11)%252C12(17)%252C13%252C15-tetraene.svg.png.webp)
-15-methyl-15-azatetracyclo(10.2.1.0%C2%B2%252C%C2%B9%E2%81%B0.0%E2%81%B4%252C%E2%81%B9)pentadeca-4(9)%252C5%252C7-trien-3-one.svg.png.webp)
(1R,2S,10R,12S)-15-methyl-15-azatetracyclo(10.2.1.02,10.04,9)pentadeca-4(9),5,7-trien-3-one[3]
C3 to 1′ + 2′ (ortho) tropane locant dual arene bridged
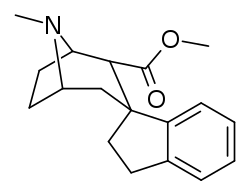
Parent compound of a series of spirocyclic cocaine benzoyl linkage modification analogs created by Suzuki coupling method of ortho-substituted arylboronic acids and an enol-triflate derived from cocaine; which technically has the three methylene length of cocaine analogues as well as the single length which defines the phenyltropane series. Note that the carbomethoxyl group is (due to constraints in synthetic processes used in the creation of this compound) alpha configured; which is not the usual, most prevalent, conformation favored for the PT cocaine-receptor binding pocket of most such sub-type of chemicals. The above and below depictions show attested compounds synthesized, additionally with variations upon the Endo–exo isomerism of their structures.[40]

Cycloalkane-ring alterations of the tropane ring system
Azanonane (outer ring extended)
3-Phenyl-9-azabicyclo[3.3.1]nonane derivatives
To better elucidate the binding requirements at MAT, the methylene unit on the tropane was extended by one to create the azanonane analogs.[lower-alpha 9] Which are the beginning of classes of modifications that start to become effected by the concerns & influences of macrocyclic stereocontrol.
Despite the loosened flexibility of the ring system, nitrogen constrained variants (such as were created to make the bridged class of phenyltropanes) which might better fit the rigid placement necessary to suit the spatial requirements needed in the binding pocket were not synthesized. Though front-bridged types were synthesized for the piperidine homologues: the trend of equal values for either isomers of that type followed the opposing trend of a smaller and lessened plasticity of the molecule to contend with a rationale for further constraining the pharmacophore within that scope. Instead such findings lend credence to the potential for the efficacy of fusing the nitrogen on an enlarged tropane, as like upon the compounds given below.
| Structure | Compound # (S. Singh) |
Ki (nM) |
|---|---|---|
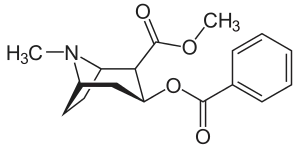 | Cocaine | 32 ± 5 390 ± 220 |
 | WIN 35065-2 | 33 ± 17 310 ± 220 |
 | 146a | 4600 ± 510 |
 | 146b | 5730 ± 570 |
 | 146c | 3450 ± 310 |
 | 146d | 3470 ± 350 |
 | 147 | 13900 ± 2010 |
Azabornane (outer ring contracted)
3-Phenyl-7-azabicyclo[2.2.1]heptane derivatives
Ring-contracted analogs of phenyltropanes did not permit sufficient penetration of the phenyl into the target binding site on MAT for an affinity in the efficacious range. The distance from the nitrogen to the phenyl centroid for 155a was 4.2 and 155c was 5.0 Å, respectively. (Whereas troparil was 5.6 & compound 20a 5.5 angstroms). However piperidine homologues (discussed below) had comparable potencies.[lower-alpha 10]
heptane.png.webp)
The non-carboxylic (and DAT substrate, releasing agent) variant of exo-2-phenyl-7-azabicyclo(2.2.1)heptane-1-carboxylic acid (N.B. the carboxy in the latter shares the C1 tropane position with the two carbon nitrogen containing bridge; sharing in the leftmost (R) substitution of the above depiction & unlike the placement on the tropane for either the carbmethoxy or phenyl ring of the azabornane analogues given in this section)
With the carboxy ester function removed the resultant derived compound acts as a DAT substrate drug, thus an amphetaminergic releaser of MAT & VMAT, yet similar to phenyltropanes (that usually are only re-uptake ligands)[41] cf. EXP-561 & BTQ.
Azabornanes with longer substitutions at the 3β-position (benzoyloxys alkylphenyls, carbamoyls etc.) or with the nitrogen in the position it would be on the piperidine homologues (i.e. arrangements of differing locations for the nitrogens being either distal or proximal within the terms required to facilitate the framework of the compound to a correlative proportion, functional for the given moiety), were not synthesized, despite conclusions that the nitrogen to phenyl length was the issue at variance enough to be the interfering factor for the proper binding of the compressed topology of the azabornane. Carroll, however, has listed benzoyloxy azabornanes in patents.[3]
| Structure | Compound # (S. Singh) |
Ki (nM) |
|---|---|---|
 | Cocaine | 32 ± 5 390 ± 220 |
 | WIN 35065-2 | 33 ± 17 310 ± 220 |
 | 155a | 60,400 ± 4,800 |
  | 155b | 96,500 ± 42 |
 | 155c | 5,620 ± 390 |
 | 155d | 18,900 ± 1,700 |
Piperidine homologues (inner two-carbon bridge excised)
Piperidine homologues had comparable affinity & potency spreads to their respective phenyltropane analogues. Without as much of a discrepancy between the differing isomers of the piperidine class with respect to affinity and binding values as had in the phenyltropanes.
p-chloro & related (piperidine homologues of phenyltropanes)
| Structure | Compound # (S. Singh) |
X = para- / 4′- Substitution |
R = 2-tropane position | DAT (IC50 nM) [H3]WIN 35428 binding displacement |
DA (IC50 nM) [H3]DA uptake |
Selectivity Uptake/Binding |
|---|---|---|---|---|---|---|
 | ||||||
| Cocaine | H | CO2Me | 102 ± 9 | 239 ± 1 | 2.3 | |
 | ||||||
| (±)-166a | Cl | β-CO2CH3 | 53.7 ± 1.9 | 37.8 ± 7.9 | 0.7 | |
| (-)-166a | Cl | β-CO2CH3 | 24.8 ± 1.6 | 85.2 ± 2.6 | 3.4 | |
| (+)-166a | Cl | β-CO2CH3 | 1360 ± 125 | 5090 ± 172 | 3.7 | |
 | ||||||
| (-)-167a | Cl | β-CO2OH | 75.3 ± 6.2 | 49.0 ± 3.0 | 0.6 | |
| (+)-167a | Cl | β-CO2OH | 442 ± 32 | — | — | |
 | ||||||
| (-)-168a | Cl | β-CO2OAc | 44.7 ± 10.5 | 62.9 ± 2.7 | 1.4 | |
| (+)-168a | Cl | β-CO2OAc | 928 ± 43 | 2023 ± 82 | 2.2 | |
 | ||||||
| (-)-169a[42] | Cl | β-n-Pr | 3.0 ± 0.5 | 8.3 ± 0.6 | 2.8 | |
 | ||||||
| (-)-170a | H | β-CO2CH3 | 769 ± 19 | — | — | |
 | ||||||
| (±)-166b | Cl | α-CO2CH3 | 197 ± 8 | — | — | |
| (+)-166b | Cl | α-CO2CH3 | 57.3 ± 8.1 | 34.6 ± 3.2 | 0.6 | |
| (-)-166b | Cl | α-CO2CH3 | 653 ± 38 | 195 ± 8 | 0.3 | |
 | ||||||
| (+)-167b | Cl | α-CO2OH | 240 ± 18 | 683 ± 47 | 2.8 | |
 | ||||||
| (+)-168b | Cl | α-CO2OAc | 461 ± 11 | — | — | |
 | ||||||
| (+)-169b | Cl | α-n-Pr | 17.2 ± 0.5 | 23.2 ± 2.2 | 1.3 |
Heterocyclic N-Desmethyl[43]
-3-(3-methyl-(1%252C2%252C4)oxadiazol-5-yl)-piperidine.png.webp)
naphthyl & related (piperidine homologues of phenyltropanes)
| Structure | Compound # | [H3]DA uptake (nM) IC50 |
[H3]DA uptake (nM) Ki |
[H3]NE uptake (nM) IC50 |
[H3]NE uptake (nM) Ki |
[H3]5-HTT uptake (nM) IC50 |
[H3]5-HTT uptake (nM) Ki |
Uptake Ratio DA/5-HT (Ki) |
Uptake Ratio NE/5-HT (Ki) |
|---|---|---|---|---|---|---|---|---|---|
 | Cocaine | 459 ± 159 | 423 ± 147 | 127 ± 4.1 | 108 ± 3.5 | 168 ± 0.4 | 155 ± 0.4 | 2.7 | 0.69 |
 | Fluoxetine | >4500 | >2500 | 193 ± 4.1 | 176 ± 3.5 | 8.1 ± 0.7 | 7.3 ± 0.7 | 624 | 24 |
 | 20 | 75 ± 9.1 | 69 ± 8.1 | 101 ± 3.3 | 88 ± 2.9 | 440 ± 30 | 391 ± 27 | 0.18 | 0.23 |
 | 6 | 23 ± 1.0 | 21 ± 0.9 | - | 34 ± 0.8 | 8.2 ± 0.3 | 7.6 ± 0.2 | 2.8 | 4.5 |
 | 7 | >1000 | 947 ± 135 | - | 241 ± 1.7 | 8.2 ± 0.3 | 7.6 ± 0.2 | 22.6 | 5.7 |
 | 8 | 94 ± 9.6 | 87 ± 8.9 | - | 27 ± 1.6 | 209 ± 17 | 192 ± 16 | 0.45 | 0.14 |
 | 9 | 293 ± 6.4 | 271 ± 5.9 | - | 38 ± 4.0 | 13 ± 0.7 | 12 ± 0.7 | 23 | 3.2 |
 | 19 | 97 ± 8.6 | 90 ± 8.0 | 34 ± 2.5 | 30 ± 2.3 | 3.9 ± 0.5 | 3.5 ± 0.5 | 26 | 8.6 |
 | 10 | 326 ± 1.2 | 304 ± 1.1 | 337 ± 37 | 281 ± 30 | 113 ± 4.3 | 101 ± 3.8 | 3.0 | 2.8 |
 | 14 | 144 ± 20 | 131 ± 18 | 204 ± 5.6 | 175 ± 4.8 | 155 ± 3.9 | 138 ± 3.5 | 0.95 | 1.3 |
 | 15 | >1800 | >1700 | >1300 | >1100 | 275 ± 39 | 255 ± 37 | >6 | >4 |
 | 16 | >1000 | 964 ± 100 | >1200 | >1000 | 334 ± 48 | 309 ± 44 | 3.1 | 3.5 |
 | 17 | 213 ± 30 | 187 ± 26 | 399 ± 12 | 364 ± 9.2 | 189 ± 37 | 175 ± 34 | 1.1 | 2.1 |
 | 18 | 184 ± 30 | 173 ± 26 | 239 ± 42 | 203 ± 36 | 67 ± 4.5 | 62 ± 4.1 | 2.8 | 3.3 |
distal-nitrogen 'dimethylamine' (piperidine-like cyclohexyl homologues of phenyltropanes)[3]



cf. Fencamfamine
Radiolabeled


| Code | SERT Ki (nM) | NET Ki (nM) | DAT Ki (nM) | Radiolabel | In vivo study | Refs. |
|---|---|---|---|---|---|---|
| 1 | 0.2 | 102.2 | 29.9 | 11C | Non-human primate | [46] |
| 2 | 0.2 | 31.7 | 32.6 | 11C | Non-human primate | [47] |
| 3 | 0.05 | 24 | 3.47 | 123I | Rat | [48] |
| 4 | 0.08 | 28 | 13 | 18F | Non-human primate | [49] |
| 5 | 0.11 | 450 | 22 | 11C | Rat, monkey | [50] |
-ene-2%CE%B2-carbomethoxy-3%CE%B2-(4%E2%80%B2-chlorophenyl)tropane.png.webp)

Transition metal complexes
These compounds include transition metals in their heteroatomic conformation, unlike non-radiolabel intended chelates where their element is chosen for intrinsic affectation to binding and function, these are tagged on by a "tail" (or similar) with a sufficient spacer to remain separated from known binding properties and instead are meant to add radioactivity enough to be easily tracked via observation methods that utilize radioactivity. As for anomalies of binding within the spectrum of the under-written kinds just mentioned: other factors not otherwise considered to account for its relatively lower potency, "compound 89c" is posited to protrude forward at the aryl place on its moiety toward the MAT ligand acceptor site in a manner detrimental to its efficacy. That is considered due to the steric bulk of the eight-position "tail" chelate substituted constituent, overreaching the means by which it was intended to be isolated from binding factors upon a tail, and ultimately nonetheless, interfering with its ability to bind. However, to broach this discrepancy, decreasing of the nitrogen tether at the eight position by a single methylene unit (89d) was shown to bring the potency of the analogous compound to the expected, substantially higher, potency: The N-methyl analog of 89c having an IC50 of 1.09 ± 0.02 @ DAT & 2.47 ± 0.14 nM @ SERT; making 89c upwards of thirty-three times weaker at those MAT uptake sites.[lower-alpha 11]
| Structure | Compound # (S. Singh) |
X = para- / 4′- Substitution |
Configuration | DAT (IC50 nM) displacement of [H3]WIN 35428 |
5-HTT (IC50 nM) [H3]Citalopram |
Selectivity 5-HTT/DAT |
|---|---|---|---|---|---|---|
 | WIN 35428 | F | - | 11.0 ± 1.0 | 160 ± 20 | 14.5 |
| +2β-chelated phenyltropanes | ||||||
 | 73 TRODAT-1ɑ | Cl | - | R=13.9, S=8.42b | - | - |
 | 74 TROTEC-1 | F | - | high affinity site = 0.15 ± 0.04c low affinity site = 20.3 ± 16.1c | - | - |
| N-chelated phenyltropanes | ||||||
 | 89a | F | 2β | 5.99 ± 0.81 | 124 ± 17 | 20.7 |
 | 89b | F | 2α | 2960 ± 157 | 5020 ± 1880 | 1.7 |
 | 89c | 3,4-Cl2 | 2β | 37.2 ± 3.4 | 264 ± 16 | 7.1 |
 | 89d | Cl | - | 0.31 ± 0.03d | - | - |
- ɑIUPAC: [2-[[2-[[[3-(4-chlorophenyl)-7-methyl-8-azabicyclo[3,2,1]oct-2-yl]methyl]-(2-mercaptoethyl)amino]ethyl]amino]ethanethiolato-(3—)-N2, N2′, S2, S2′]oxo-[1''R''-(''exo'', ''exo'')]-[99mTc]technetium
- bR- & S- isomer values are Ki (nM) for displacement of [125I]IPT with technetium-99m replaced by rhenium
- cIC50 (nM) values for displacement of [3H]WIN 35428 with ligand tricarbonyltechnetium replaced with rhenium. (IC50 for WIN 35428 were 2.62 ± 1.06 @ high affinity binding & 139 ± 72 @ low affinity binding sites)
- dKi value for displacement of [125I]IPT radioligand.
Select annotations of above
Phenyltropanes can be grouped by "N substitution" "Stereochemistry" "2-substitution" & by the nature of the 3-phenyl group substituent X.
Often this has dramatic effects on selectivity, potency, and duration, also toxicity, since phenyltropanes are highly versatile. For more examples of interesting phenyltropanes, see some of the more recent patents, e.g. U.S. Patent 6,329,520, U.S. Patent 7,011,813, U.S. Patent 6,531,483, and U.S. Patent 7,291,737.
Potency in vitro should not be confused with the actual dosage, as pharmacokinetic factors can have a dramatic influence on what proportion of an administered dose actually gets to the target binding sites in the brain, and so a drug that is very potent at binding to the target may nevertheless have only moderate potency in vivo. For example, RTI-336 requires a higher dosage than cocaine. Accordingly, the active dosage of RTI-386 is exceedingly poor despite the relatively high ex vivo DAT binding affinity.
Sister substances
Many molecular drug structures have exceedingly similar pharmarcology to phenyltropanes, yet by certain technicalities do not fit the phenyltropane moniker. These are namely classes of dopaminergic cocaine analogues that are in the piperidine class (a category that includes methylphenidate) or benztropine class (such as Difluoropine: which is extremely close to fitting the criteria of being a phenyltropane.) Whereas other potent DRIs are far removed from being in the phenyltropane structural family, such as Benocyclidine or Vanoxerine.
See: List of cocaine analogues
Most any variant with a tropane locant—3-β (or α) connecting linkage differing from, e.g. longer than, a single methylene unit (i.e. "phenyl"), including alkylphenyls (see the styrene analog, first image given in example below) is more correctly a "cocaine analogue" proper, and not a phenyltropane. Especially if this linkage imparts a sodium channel blocker functionality to the molecule:








| More cocaine analogue examples |
|---|
|
|
See also

References
Citations
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 Singh, Satendra (2000). "Chem Inform Abstract: Chemistry, Design, and Structure-Activity Relationship of Cocaine Antagonists" (PDF). ChemInform. 31 (20): no. doi:10.1002/chin.200020238. Mirror hotlink.
- ↑ U.S. Patent Application Publication # US 2008/0153870 A1 M. J. Kuhar, et al. Jun. 26, 2008. Research Triangle Institute.
- 1 2 3 4 5 6 7 8 9 10 U.S. Patent 6,479,509
- 1 2 3 Tamagnan, Gilles (2005). "Synthesis and monoamine transporter affinity of new 2β-carbomethoxy-3β-[4-(substituted thiophenyl)]phenyltropanes: discovery of a selective SERT antagonist with picomolar potency". Bioorganic & Medicinal Chemistry. 15 (4): 1131–1133. doi:10.1016/j.bmcl.2004.12.014. PMID 15686927.
- ↑ Schmitt, K. C.; Rothman, R. B.; Reith, M. E. (Jul 2013). "Nonclassical Pharmacology of the Dopamine Transporter: Atypical Inhibitors, Allosteric Modulators, and Partial Substrates". J Pharmacol Exp Ther. 346 (1): 2–10 Fig. 1. doi:10.1124/jpet.111.191056. PMC 3684841. PMID 23568856.
- ↑ U.S. Patent 6,479,509 Method of promoting smoking cessation.
- ↑ Blough, B. E.; Keverline, K. I.; Nie, Z.; Navarro, H.; Kuhar, M. J.; Carroll, F. I. (2002). "Synthesis and transporter binding properties of 3β-4′-(phenylalkyl, -phenylalkenyl, and -phenylalkynyl)phenyltropane-2β-carboxylic acid methyl esters: evidence of a remote phenyl binding domain on the dopamine transporter". Journal of Medicinal Chemistry. 45 (18): 4029–4037. doi:10.1021/jm020098n. PMID 12190324.
- 1 2 Blough, Bruce E.; Keverline, Kathryn I.; Nie, Zhe; Navarro, Hernán; Kuhar, Michael J.; Carroll, F. Ivy (2002). "Synthesis and Transporter Binding Properties of 3β-[4'-(Phenylalkyl, -phenylalkenyl, and -phenylalkynl)phenyl]tropane-2β-carboxylic Acid Methyl Esters: Evidence of a Remote Phenyl Binding Domain on the Dopamine Transporter". Journal of Medicinal Chemistry. 45 (18): 4029–37. doi:10.1021/jm020098n. PMID 12190324.
- ↑ Blough; et al. (Sep 1996). "Synthesis and transporter binding properties of 3β-(4'-alkyl-, 4'-alkenyl-, and 4'-alkynylphenyl)nortropane-2 β-carboxylic acid methyl esters: serotonin transporter selective analogs". J Med Chem. 39 (20): 4027–35. doi:10.1021/jm960409s. PMID 8831768. S2CID 21616809.
- 1 2 Meltzer, P. C.; Liang, A. Y.; Brownell, A. L.; Elmaleh, D. R.; Madras, B. K. (1993). "Substituted 3-phenyltropane analogs of cocaine: Synthesis, inhibition of binding at cocaine recognition sites, and positron emission tomography imaging". Journal of Medicinal Chemistry. 36 (7): 855–62. doi:10.1021/jm00059a010. PMID 8464040.
- 1 2 Meltzer, P. C.; McPhee, M.; Madras, B. K. (2003). "Synthesis and biological activity of 2-Carbomethoxy-3-catechol-8-azabicyclo[3.2.1]octanes". Bioorganic & Medicinal Chemistry Letters. 13 (22): 4133–4137. doi:10.1016/j.bmcl.2003.07.014. PMID 14592523.
- ↑ R. H. Kline, Davies, E. Saikali, T. Sexton & S.R. Childers (1993). "Novel 2-substituted cocaine analogs: Binding properties at dopamine transport sites in rat striatum". European Journal of Pharmacology. 244 (1): 93–97. doi:10.1016/0922-4106(93)90063-f. PMID 8420793.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Jin, C; Navarro, H. A.; Carroll, F. I. (2008). "Development of 3-Phenyltropane Analogs with High Affinity for the Dopamine and Serotonin Transporters and Low Affinity for the Norepinephrine Transporter". Journal of Medicinal Chemistry. 51 (24): 8048–8056. doi:10.1021/jm801162z. PMC 2841478. PMID 19053748. Table 1.
- ↑ Jin, C; Navarro, H. A.; Carroll, F. I. (2008). "Development of 3-Phenyltropane Analogs with High Affinity for the Dopamine and Serotonin Transporters and Low Affinity for the Norepinephrine Transporter". Journal of Medicinal Chemistry. 51 (24): 8048–8056. doi:10.1021/jm801162z. PMC 2841478. PMID 19053748. Table 2.
- ↑ Zhong, Desong; Kotian, Pravin; Wyrick, Christopher D.; Seltzman, Herbert H.; Kepler, John A.; Kuhar, Michael J.; Boja, John W.; Carroll, F. Ivy (1999). "Synthesis of 3β-(4-[125I]iodophenyl)tropane-2-β-pyrrolidine carboxamide ([125I]RTI-229)". Journal of Labelled Compounds and Radiopharmaceuticals. 42 (3): 281–286. doi:10.1002/(SICI)1099-1344(199903)42:3<281::AID-JLCR188>3.0.CO;2-X.
- ↑ Carroll, F. I.; Gray; Abraham; Kuzemko; Lewin; Boja; Kuhar (1993). "3-Aryl-2-(3′-substituted-1′,2′,4'-oxadiazol-5′-yl)tropane analogues of cocaine: affinities at the cocaine binding site at the dopamine, serotonin, and norepinephrine transporters". Journal of Medicinal Chemistry. 36 (20): 2886–2890. doi:10.1021/jm00072a007. PMID 8411004.
- 1 2 Methods for controlling invertebrate pests using cocaine receptor binding ligands. U.S. Patent 5,935,953
- ↑ Carroll, F.; Howard, J.; Howell, L.; Fox, B.; Kuhar, M. (2006). "Development of the dopamine transporter selective RTI-336 as a pharmacotherapy for cocaine abuse". The AAPS Journal. 8 (1): E196–E203. doi:10.1208/aapsj080124. PMC 2751440. PMID 16584128.
- ↑ Carroll, F.; Howard, J.; Howell, L.; Fox, B.; Kuhar, M. (2006). "Development of the dopamine transporter selective RTI-336 as a pharmacotherapy for cocaine abuse". The AAPS Journal. 8 (1): E196–E203. doi:10.1208/aapsj080124. PMC 2751440. PMID 16584128.
- ↑ Davies, Huw M.L; Ren, Pingda; Kong, Norman; Sexton, Tammy; Childers, Steven R (2001). "Synthesis and monoamine transporter affinity of 3β-(4-(2-pyrrolyl)phenyl)-8-azabicyclo[3.2.1]octanes and 3β-(5-Indolyl)-8-azabicyclo[3.2.1]octanes". Bioorganic & Medicinal Chemistry Letters. 11 (4): 487–489. doi:10.1016/S0960-894X(00)00701-0. ISSN 0960-894X. PMID 11229754.
- ↑ Davies, H. M.; Gilliatt, V; Kuhn, L. A.; Saikali, E; Ren, P; Hammond, P. S.; Sexton, T; Childers, S. R. (2001). "Synthesis of 2β-Acyl-3β-(substituted naphthyl)-8-azabicyclo[3.2.1]octanes and Their Binding Affinities at Dopamine and Serotonin Transport Sites". Journal of Medicinal Chemistry. 44 (10): 1509–1515. doi:10.1021/jm000363+. PMID 11334561.
- ↑ Carroll, F. I.; Gao; Abraham; Lewin; Lew; Patel; Boja; Kuhar (1992). "Probes for the cocaine receptor. Potentially irreversible ligands for the dopamine transporter". Journal of Medicinal Chemistry. 35 (10): 1813–1817. doi:10.1021/jm00088a017. PMID 1588560.
- ↑ Wu; Reith, M.; Walker, Q.; Kuhn, C.; Carroll, F.; Garris, P. (2002). "Concurrent autoreceptor-mediated control of dopamine release and uptake during neurotransmission: an in vivo voltammetric study". Journal of Neuroscience. 22 (14): 6272–6281. doi:10.1523/JNEUROSCI.22-14-06272.2002. PMC 6757948. PMID 12122086.
- ↑ Murthy, V; Martin, TJ; Kim, S; Davies, HM; Childers, SR (August 2008). "In vivo characterization of a novel phenylisothiocyanate tropane analog at monoamine transporters in rat brain". J. Pharmacol. Exp. Ther. 326 (2): 587–95. doi:10.1124/jpet.108.138842. PMID 18492949. S2CID 5996473.
- ↑ Xu, L.; Kulkarni, S. S.; Izenwasser, S.; Katz, J. L.; Kopajtic, T.; Lomenzo, S. A.; Newman, A. H.; Trudell, M. L. (2004). "Synthesis and Monoamine Transporter Binding of 2-(Diarylmethoxymethyl)-3β-aryltropane Derivatives". Journal of Medicinal Chemistry. 47 (7): 1676–82. doi:10.1021/jm030430a. PMID 15027858.
- ↑ Hong, W. C.; Kopajtic, T. A.; Xu, L.; Lomenzo, S. A.; Jean, B.; Madura, J. D.; Surratt, C. K.; Trudell, M. L.; Katz, J. L. (2016). "2-Substituted 3 -Aryltropane Cocaine Analogs Produce Atypical Effects without Inducing Inward-Facing Dopamine Transporter Conformations". Journal of Pharmacology and Experimental Therapeutics. 356 (3): 624–634. doi:10.1124/jpet.115.230722. ISSN 1521-0103. PMC 4767397. PMID 26769919. nih.gov article (inclu. structural depictions)
- ↑ Cesati, RR 3rd; Tamagnan, G; Baldwin, RM; Zoghbi, SS; Innis, RB; Kula, NS; Baldessarini, RJ; Katzenellenbogen, JA (2002). "Synthesis of cyclopentadienyltricarbonyl rhenium phenyltropanes by double ligand transfer: organometallic ligands for the dopamine transporter". Bioconjug Chem. 13 (1): 29–39. doi:10.1021/bc010011x. PMID 11792176.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ↑ Bloom, Jacob W. G.; Wheeler, Steven E. (2011). "Taking the Aromaticity out of Aromatic Interactions". Angew. Chem. 123 (34): 7993–7995. Bibcode:2011AngCh.123.7993B. doi:10.1002/ange.201102982.
- ↑ A novel spirocyclic tropanyl-Δ2-isoxazoline derivative enhances citalopram and paroxetine binding to serotonin transporters as well as serotonin uptake. Bioorg Med Chem 2012 Nov 10;20(21):6344-55. Epub 2012 Sep 10.
- ↑ Hanna, Mona M. (2007). "Synthesis of some tropane derivatives of anticipated activity on the reuptake of norepinephrine and/or serotonin". Bioorganic. 15 (24): 7765–7772. doi:10.1016/j.bmc.2007.08.055. PMID 17870537.
- ↑ Goodman, Mark M. (2003). "Synthesis and Characterization of Iodine-123 Labeled 2β-Carbomethoxy-3β-(4′-((Z)-2-iodoethenyl)phenyl)nortropane. A Ligand for in Vivo Imaging of Serotonin Transporters by Single-Photon-Emission Tomography". Journal of Medicinal Chemistry. 46 (6): 925–935. doi:10.1021/jm0100180. PMID 12620070.
- ↑ Blough, B.; Abraham, P.; Lewin, A.; Kuhar, M.; Boja, J.; Carroll, F. (1996). "Synthesis and transporter binding properties of 3β-(4′-alkyl-, 4′-alkenyl-, and 4′-alkynylphenyl)nortropane-2β-carboxylic acid methyl esters: serotonin transporter selective analogs". Journal of Medicinal Chemistry. 39 (20): 4027–4035. doi:10.1021/jm960409s. PMID 8831768. S2CID 21616809.
- ↑ Spealman, R. D.; Kelleher, R. T. (Mar 1981). "Self-administration of cocaine derivatives by squirrel monkeys". The Journal of Pharmacology and Experimental Therapeutics. 216 (3): 532–536. ISSN 0022-3565. PMID 7205634.
- ↑ Carroll, F.; Tyagi, S.; Blough, B.; Kuhar, M.; Navarro, H. (2005). "Synthesis and monoamine transporter binding properties of 3α-(substituted phenyl)nortropane-2β-carboxylic acid methyl esters. Norepinephrine transporter selective compounds". Journal of Medicinal Chemistry. 48 (11): 3852–3857. doi:10.1021/jm058164j. PMID 15916437.
- ↑ Purushotham, M; Sheri, A; Pham-Huu, D. P.; Madras, B. K.; Janowsky, A; Meltzer, P. C. (2011). "The synthesis and biological evaluation of 2-(3-methyl or 3-phenylisoxazol-5-yl)-3-aryl-8-thiabicyclo3.2.1octanes". Bioorganic & Medicinal Chemistry Letters. 21 (1): 48–51. doi:10.1016/j.bmcl.2010.11.076. PMC 3015105. PMID 21146984.
- 1 2 Wu, Xiaoai; Cai, Huawei; Ge, Ran; Li, Lin; Jia, Zhiyun (2015). "Recent Progress of Imaging Agents for Parkinson's Disease". Current Neuropharmacology. 12 (6): 551–563. doi:10.2174/1570159X13666141204221238. ISSN 1570-159X. PMC 4428027. PMID 25977680.
- ↑ U.S. Patent 6,150,376
- ↑ WO 0007994, Kozikowski, Alan P. & Smith, Miles P., "Novel bi- and tri-cyclic aza compounds and their uses", published 2000-02-17, assigned to Georgetown University
- ↑ Sakamuri, Sukumar; et al. (2000). "Synthesis of novel spirocyclic cocaine analogs using the Suzuki coupling". Tetrahedron Letters. 41 (13): 2055–2058. doi:10.1016/S0040-4039(00)00113-1.
- ↑ exo-2-Phenyl-7-azabicyclo[2.2.1]heptane-1-carboxylic Acid: A New Constrained Proline Analogue. Source: Tetrahedron Letters, Volume 36, Number 39, 25 September 1995, pp. 7123-7126(4)
- ↑ Kozikowski, A. P.; Araldi, G. L.; Boja, J.; Meil, W. M.; Johnson, K. M.; Flippen-Anderson, J. L.; George, C.; Saiah, E. (1998). "Chemistry and Pharmacology of the Piperidine-Based Analogues of Cocaine. Identification of Potent DAT Inhibitors Lacking the Tropane Skeleton". Journal of Medicinal Chemistry. 41 (11): 1962–9. CiteSeerX 10.1.1.512.7158. doi:10.1021/jm980028+. PMID 9599245.
- ↑ NIH U.S. National Library of Medicine. PubChem CID: 44337825, InChI Key: MHDRABCQAWNSIK-PZORYLMUSA-N
- ↑ Further SAR Studies of Piperidine-Based Analogues of Cocaine. 2. Potent Dopamine and Serotonin Reuptake Inhibitors J. Med. Chem. 2000,43,1215-1222
- ↑ Napier, Susan; Bingham, Matilda (2009). Transporters as Targets for Drugs. Topics in Medicinal Chemistry. Vol. 4. Bibcode:2009ttd..book.....N. doi:10.1007/978-3-540-87912-1. ISBN 978-3-540-87911-4.
- ↑ Stehouwer, Jeffrey S. (2006). "Synthesis, Radiosynthesis, and Biological Evaluation of Carbon-11 Labeled 2β-Carbomethoxy-3β-(3′-(( Z )-2-haloethenyl)phenyl)nortropanes: Candidate Radioligands for in Vivo Imaging of the Serotonin Transporter with Positron Emission Tomography". Journal of Medicinal Chemistry. 49 (23): 6760–6767. doi:10.1021/jm060641q. PMID 17154506.
- ↑ Deskus, Jeffrey A. (2007). "Conformationally restricted homotryptamines 3. Indole tetrahydropyridines and cyclohexenylamines as selective serotonin reuptake inhibitors". Bioorganic & Medicinal Chemistry. 17 (11): 3099–3104. doi:10.1016/j.bmcl.2007.03.040. PMID 17391962.
- ↑ Schmitz, William D. (2005). "Homotryptamines as potent and selective serotonin reuptake inhibitors (SSRIs)". Bioorganic & Medicinal Chemistry. 15 (6): 1619–1621. doi:10.1016/j.bmcl.2005.01.059. PMID 15745809.
- ↑ Plisson, Christophe (2007). "Synthesis and in Vivo Evaluation of Fluorine-18 and Iodine-123 Labeled 2β-Carbo(2-fluoroethoxy)-3β-(4′-(( Z )-2-iodoethenyl)phenyl)nortropane as a Candidate Serotonin Transporter Imaging Agent". Journal of Medicinal Chemistry. 50 (19): 4553–4560. doi:10.1021/jm061303s. PMID 17705359.
- ↑ McMahon, C. G.; McMahon, C. N.; Leow, L. J. (2006). "New agents in the treatment of premature ejaculation". Neuropsychiatric Disease and Treatment. 2 (4): 489–503. doi:10.2147/nedt.2006.2.4.489. PMC 2671940. PMID 19412497.
- ↑ Leung, K (2004). "N-4-Fluorobut-2-yn-1-yl-2β-carbo-[11C]methoxy-3β-phenyltropane". PMID 22073420.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Stenzinger, W; Blömker, A; Hiddemann, W; de Loo, J (1990). "Treatment of refractory multiple myeloma with the vincristine-adriamycin-dexamethasone (VAD) regimen". Blut. 61 (2–3): 55–9. doi:10.1007/bf02076700. PMID 2207342. S2CID 25860357.
- ↑ Ma, S; Cheng, MH; Guthrie, DA; Newman, AH; Bahar, I; Sorkin, A (2017). "Targeting of dopamine transporter to filopodia requires an outward-facing conformation of the transporter". Sci Rep. 7 (1): 5399. Bibcode:2017NatSR...7.5399M. doi:10.1038/s41598-017-05637-x. PMC 5511133. PMID 28710426.
Im-pact indices (exact locations within sources cited) & foot-notations
- ↑ [1] ←Page #929 (5th page of article) § II
- ↑ Many of the RTI phenyltropanes are "RTI-4229-×××" where × is the specific phenyltropane code number.
—
e.g. RTI-55 is in-fact RTI-4229-55 but given below as simply RTI-55 for the sake of simplicity in shorthand (following as is done in the literature itself) as the subject matter in context is wholly within the scope of the phenyltropane coded category herein. Sometimes (more rarely) it is given as RTI-COC-××× for "cocaine derivative."
—
Worth mentioning in notation as to explain that other compounds entirely unrelated can be found with the same "RTI-×××" short-numbered assignation. Therefore it is to be expected that within different contexts a compound or chemical of the same name very possibly could be in reference to a entirely other substance of another chemical series non-analogous to those in this topic. - ↑ [1] ←Page #970 (46th page of article) §B, 10th line
- ↑ [1] ←Page #971 (47th page of article) 1st ¶, 10th line
- ↑ Beta (i.e. 2,3 Rectus)-Carbmethoxy-Phenyl-Tropane
- ↑ Beta (i.e. 2,3 Rectus)-Carbmethoxy-Fluorophenyl-Tropane
- ↑ [1] ←Page #940 (16th page of article) underneath Table 8., above § 4
- ↑ [1] ←Page #941 (17th page of article) Figure 10
- ↑ [1] ←Page #967 (43rd page of article) 2nd column
- ↑ [1] ←Page #967 (43rd page of article) 2nd column
- ↑ [1] ←Page #955 (31st page of article) 1st (left) column, 2nd ¶
External links
- U. S. Provisional Patent Application listing examples of compounds that are tropanes for prospective use in research
- Article on cocaine analogue research Archived 2006-09-22 at the Wayback Machine