-chlorid.png.webp) | |
_chloride_tetrahydrate.jpg.webp) Tetrahydrate | |
| Names | |
|---|---|
| Other names
Indium chloride Indium trichloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.027 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 3260 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| InCl3 | |
| Molar mass | 221.18 g/mol |
| Appearance | white flakes |
| Density | 3.46 g/cm3 |
| Melting point | 586 °C (1,087 °F; 859 K) |
| Boiling point | 800 °C (1,470 °F; 1,070 K) |
| 195 g/100 mL, exothermic | |
| Solubility in other solvents | THF, Ethanol |
| Structure | |
| Monoclinic, mS16 | |
| C12/m1, No. 12 | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Corrosive |
| GHS labelling: | |
  [1] [1] | |
| Danger[1] | |
| H302, H314[1] | |
| P260, P301+P330+P331, P303+P361+P353, P305+P351+P338, P405, P501[1] | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | External SDS |
| Related compounds | |
Other anions |
Indium(III) fluoride Indium(III) bromide Indium(III) iodide |
Other cations |
Aluminium chloride Gallium trichloride Thallium(III) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Indium(III) chloride is the chemical compound with the formula InCl3 which forms a tetrahydrate. This salt is a white, flaky solid with applications in organic synthesis as a Lewis acid. It is also the most available soluble derivative of indium.[2] This is one of three known indium chlorides.
Synthesis and structure
Being a relatively electropositive metal, indium reacts quickly with chlorine to give the trichloride. Indium trichloride is very soluble and deliquescent.[3] A synthesis has been reported using an electrochemical cell in a mixed methanol-benzene solution.[4]
Like AlCl3 and TlCl3, InCl3 crystallizes as a layered structure consisting of a close-packed chloride arrangement containing layers of octahedrally coordinated In(III) centers,[5] a structure akin to that seen in YCl3.[6] In contrast, GaCl3 crystallizes as dimers containing Ga2Cl6.[6] Molten InCl3 conducts electricity,[5] whereas AlCl3 does not as it converts to the molecular dimer, Al2Cl6.[7]
Reactions
InCl3 is a Lewis acid and forms complexes with donor ligands, L, InCl3L, InCl3L2, InCl3L3. For example, with the chloride ion it forms tetrahedral InCl4−, trigonal bipyramidal InCl52−, and octahedral InCl63−.[5]
In diethyl ether solution, InCl3 reacts with lithium hydride, LiH, to form . This unstable compound decomposes below 0 °C,[8] and is reacted in situ in organic synthesis as a reducing agent[9] and to prepare tertiary amine and phosphine complexes of InH3.[10]
Trimethylindium, InMe3, can be produced by reacting InCl3 in diethyl ether solution either with the Grignard reagent or methyllithium, LiMe. Triethylindium can be prepared in a similar fashion but with the grignard reagent EtMgBr.[11]
InCl3 reacts with indium metal at high temperature to form the lower valent indium chlorides In5Cl9, In2Cl3 and InCl.[5]
Catalyst in chemistry
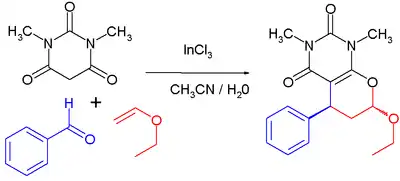
Indium chloride is a Lewis acid catalyst in organic reactions such as Friedel-Crafts acylations and Diels-Alder reactions. As an example of the latter,[12] the reaction proceeds at room temperature, with 1 mole% catalyst loading in an acetonitrile-water solvent mixture. The first step is a Knoevenagel condensation between the barbituric acid and the aldehyde; the second step is a reverse electron-demand Diels-Alder reaction, which is a multicomponent reaction of N,N'-dimethyl-barbituric acid, benzaldehyde and ethyl vinyl ether. With the catalyst, the reported chemical yield is 90% and the percentage trans isomer is 70%. Without the catalyst added, the yield drops to 65% with 50% trans product.
References
- 1 2 3 4 "Indium(III) Chloride". American Elements. Retrieved May 15, 2019.
- ↑ Araki, S.; Hirashita, T. "Indium trichloride" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289X.
- ↑ Indium Trichloride
- ↑ Habeeb, J. J.; Tuck, D. G. "Electrochemical Synthesis of Indium(III) Complexes" Inorganic Syntheses, 1979, volume XIX, ISBN 0-471-04542-X
- 1 2 3 4 Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0123526515
- 1 2 Wells, A.F. Structural Inorganic Chemistry, Oxford: Clarendon Press, 1984. ISBN 0-19-855370-6.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Anthony John Downs (1993). Chemistry of aluminium, gallium, indium, and thallium. Springer. ISBN 0-7514-0103-X.
- ↑ Main Group Metals in Organic Synthesis vol 1, ed. Hisashi Yamamoto, Koichiro Oshima, Wiley VCH, 2004, ISBN 3527305084
- ↑ The Group 13 Metals Aluminium, Gallium, Indium and Thallium: Chemical Patterns and Peculiarities, Simon Aldridge, Anthony J. Downs, Wiley, 2011, ISBN 978-0-470-68191-6
- ↑ Main Group compounds in Inorganic Syntheses, vol 31, By Schultz, Neumayer, Marks; Ed., Alan H. Cowley, John Wiley & Sons, Inc., 1997, ISBN 0471152889
- ↑ An efficient synthesis of novel pyrano[2,3-d]- and furopyrano[2,3-d]pyrimidines via Indium-Catalyzed Multicomponent Domino Reaction Prajapati, D. Mukut Gohain, M. Beilstein Journal of Organic Chemistry 2006, 2:11 doi:10.1186/1860-5397-2-11