| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name
Cadmium dichloride | |||
| Other names
Cadmium(II) chloride | |||
| Identifiers | |||
| |||
3D model (JSmol) |
| ||
| 3902835 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.030.256 | ||
| EC Number |
| ||
| 912918 | |||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII |
| ||
| UN number | 2570 | ||
CompTox Dashboard (EPA) |
| ||
| |||
| |||
| Properties | |||
| CdCl2 | |||
| Molar mass | 183.31 g·mol−1 | ||
| Appearance | White solid, hygroscopic | ||
| Odor | Odorless | ||
| Density | 4.047 g/cm3 (anhydrous)[1] 3.26 g/cm3 (monohydrate) 3.327 g/cm3 (Hemipentahydrate)[2] | ||
| Melting point | 568 °C (1,054 °F; 841 K) [2] | ||
| Boiling point | 964 °C (1,767 °F; 1,237 K) [2] | ||
| Hemipentahydrate: 79.5 g/100 mL (−10 °C) 90 g/100 mL (0 °C) Monohydrate: 119.6 g/100 mL (25 °C)[2] 134.3 g/100 mL (40 °C) 134.2 g/100 mL (60 °C) 147 g/100 mL (100 °C)[3] | |||
| Solubility | Soluble in alcohol, selenium(IV) oxychloride, benzonitrile Insoluble in ether, acetone[1] | ||
| Solubility in pyridine | 4.6 g/kg (0 °C) 7.9 g/kg (4 °C) 8.1 g/kg (15 °C) 6.7 g/kg (30 °C) 5 g/kg (100 °C)[1] | ||
| Solubility in ethanol | 1.3 g/100 g (10 °C) 1.48 g/100 g (20 °C) 1.91 g/100 g (40 °C) 2.53 g/100 g (70 °C)[1] | ||
| Solubility in dimethyl sulfoxide | 18 g/100 g (25 °C)[1] | ||
| Vapor pressure | 0.01 kPa (471 °C) 0.1 kPa (541 °C)[2] | ||
| −6.87·10−5 cm3/mol[2] | |||
| Viscosity | 2.31 cP (597 °C) 1.87 cP (687 °C)[1] | ||
| Structure | |||
| Rhombohedral, hR9 (anhydrous)[4] Monoclinic (hemipentahydrate)[3] | |||
| R3m, No. 166 (anhydrous)[4] | |||
| 3 2/m (anhydrous)[4] | |||
α = 90°, β = 90°, γ = 120° | |||
| Thermochemistry | |||
Heat capacity (C) |
74.7 J/mol·K[2] | ||
Std molar entropy (S⦵298) |
115.3 J/mol·K[2] | ||
Std enthalpy of formation (ΔfH⦵298) |
−391.5 kJ/mol[2] | ||
Gibbs free energy (ΔfG⦵) |
−343.9 kJ/mol[2] | ||
| Hazards | |||
| GHS labelling: | |||
   [5] [5] | |||
| Danger | |||
| H301, H330, H340, H350, H360, H372, H410[5] | |||
| P210, P260, P273, P284, P301+P310, P310[5] | |||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
94 mg/kg (rats, oral)[1] 60 mg/kg (mouse, oral) 88 mg/kg (rat, oral)[6] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
[1910.1027] TWA 0.005 mg/m3 (as Cd)[7] | ||
REL (Recommended) |
Ca[7] | ||
IDLH (Immediate danger) |
Ca [9 mg/m3 (as Cd)][7] | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Other anions |
Cadmium fluoride Cadmium bromide Cadmium iodide | ||
Other cations |
Zinc chloride Mercury(II) chloride Calcium chloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Cadmium chloride is a white crystalline compound of cadmium and chloride, with the formula CdCl2. This salt is a hygroscopic solid that is highly soluble in water and slightly soluble in alcohol. The crystal structure of cadmium chloride (described below), is a reference for describing other crystal structures. Also known are CdCl2•H2O and the hemipentahydrate CdCl2•2.5H2O.[2]
Structure
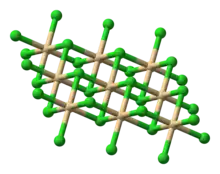
Anhydrous
Anhydrous cadmium chloride forms a layered structure consisting of octahedral Cd2+ centers linked with chloride ligands. Cadmium iodide, CdI2, has a similar structure, but the iodide ions are arranged in a HCP lattice, whereas in CdCl2 the chloride ions are arranged in a CCP lattice.[8][9]
Hydrates
The anhydrous form absorbs moisture from the air to form various hydrates. Three of these hydrates have been examined by X-ray crystallography.
| Compound | CdCl2·H2O[10] | CdCl2·2.5H2O[11] | CdCl2·4H2O[12] |
|---|---|---|---|
| Molar mass (g/mol) | 201.33 | 228.36 | 255.38 |
| Crystal Structure | Orthorhombic | Monoclinic | Orthorhombic |
| Space Group | Pnma | P21/n | P212121 |
| Lattice constant a (Å) | 9.25 | 9.21 | 12.89 |
| Lattice constant b (Å) | 3.78 | 11.88 | 7.28 |
| Lattice constant c (Å) | 11.89 | 10.08 | 15.01 |
| β | 93.5° | ||
| Density (g/cm3) | 3.26 | 2.84 | 2.41 |
| Comment | Interconnected CdCl3(H2O) octahederons | Distorted trans-[CdCl2(H2O)4] octahedrons |
Chemical properties
Cadmium chloride dissolves well in water and other polar solvents. It is a mild Lewis acid.[8]
- CdCl2 + 2 Cl− → [CdCl4]2−
Solutions of equimolar cadmium chloride and potassium chloride give potassium cadmium trichloride.[13] With large cations, it is possible to isolate the trigonal bipyramidal [CdCl5]3− ion.
Cadmium metal is soluble in molten cadmium chloride, produced by heating cadmium chloride above 568 °C. Upon cooling, the metal precipitates.[14]
Preparation
Anhydrous cadmium chloride can be prepared by the reaction of hydrochloric acid and cadmium metal or cadmium oxide.[14]
- Cd + 2 HCl → CdCl2 + H2
The anhydrous salt can also be prepared from anhydrous cadmium acetate using hydrogen chloride or acetyl chloride.[15]
Industrially, it is produced by the reaction of molten cadmium and chlorine gas at 600 °C.[14]
The monohydrate, hemipentahydrate, and tetrahydrate can be produced by evaporation of the solution of cadmium chloride at 35, 20, and 0 °C respectively. The hemipentahydrate and tetrahydrate release water in air.[10][11][12]
Uses
Cadmium chloride is used for the preparation of cadmium sulfide, used as "cadmium yellow", a brilliant-yellow stable inorganic pigment.[14]
- CdCl
2 + H
2S → CdS + 2 HCl
In the laboratory, anhydrous CdCl2 can be used for the preparation of organocadmium compounds of the type R2Cd, where R is an aryl or a primary alkyl. These were once used in the synthesis of ketones from acyl chlorides:[16]
- CdCl
2 + 2 RMgX → R
2Cd + MgCl
2 + MgX
2
- R
2Cd + 2R'COCl → 2R'COR + CdCl
2
Such reagents have largely been supplanted by organocopper compounds, which are much less toxic.
Cadmium chloride is also used for photocopying, dyeing and electroplating.
Like all cadmium compounds, CdCl
2 is highly toxic and appropriate safety precautions must be taken when handling it.
References
- 1 2 3 4 5 6 7 Anatolievich, Kiper Ruslan. "cadmium chloride". chemister.ru. Retrieved 2014-06-25.
- 1 2 3 4 5 6 7 8 9 10 11 Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- 1 2 Seidell, Atherton; Linke, William F. (1919). Solubilities of Inorganic and Organic Compounds (2nd ed.). New York: D. Van Nostrand Company. p. 169.
- 1 2 3 4 "Cadmium Chloride - CdCl2". chem.uwimona.edu.jm. Mona, Jamaica: The University of the West Indies. Retrieved 2014-06-25.
- 1 2 3 Sigma-Aldrich Co., Cadmium chloride. Retrieved on 2014-05-23.
- ↑ "Cadmium compounds (as Cd)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 NIOSH Pocket Guide to Chemical Hazards. "#0087". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997.
- ↑ A. F. Wells, Structural Inorganic Chemistry, 5th ed., Oxford University Press, Oxford, UK, 1984.
- 1 2 H. Leligny; J. C. Monier (1974). "Structure cristalline de CdCl2.H2O" [Crystal structure of CdCl2.H2O]. Acta Crystallographica B (in French). 30 (2): 305–309. doi:10.1107/S056774087400272X.
- 1 2 H. Leligny; J. C. Monier (1975). "Structure de CdCl2.2,5H2O" [Structure of CdCl2.2,5H2O]. Acta Crystallographica B (in French). 31 (3): 728–732. doi:10.1107/S056774087500369X.
- 1 2 H. Leligny; J. C. Monier (1979). "Structure de dichlorure de cadmium tétrahydraté" [Structure of cadmium dichloride tetrahydrate]. Acta Crystallographica B (in French). 35 (3): 569–573. doi:10.1107/S0567740879004179.
- ↑ F. Wagenknecht; R. Juza (1963). "Potassium cadmium chloride". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 2. NY, NY: Academic Press. p. 1095.
- 1 2 3 4 Karl-Heinz Schulte-Schrepping; Magnus Piscator (2000). "Cadmium and Cadmium Compounds". Ullmann's Encyclopedia of Industrial Chemistry (6th ed.). p. 472. doi:10.1002/14356007.a04_499. ISBN 9783527306732.
- ↑ F. Wagenknecht; R. Juza (1963). "Cadmium chloride". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 2. NY, NY: Academic Press. pp. 1093–4.
- ↑ J. March, Advanced Organic Chemistry, 4th ed., p. 723, Wiley, New York, 1992.
External links
- International Chemical Safety Card 0116
- IARC Monograph "Cadmium and Cadmium Compounds"
- National Pollutant Inventory - Cadmium and compounds