 | |
| Names | |
|---|---|
| IUPAC name
2-N-[(1R,2S)-2,6-dimethyl-2,3-dihydro-1H-inden-1-yl]-6-(1-fluoroethyl)-1,3,5-triazine-2,4-diamine[1] | |
| Identifiers | |
3D model (JSmol) |
|
| 20920435 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.216.692 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties[2] | |
| C16H20FN5 | |
| Molar mass | 301.369 g·mol−1 |
| Density | 1.23 g/mL |
| Melting point | 183 °C (361 °F; 456 K) |
| 2.8 mg/L (20 °C) | |
| log P | 2.8 |
| Hazards | |
| GHS labelling: | |
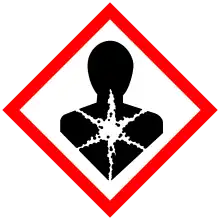 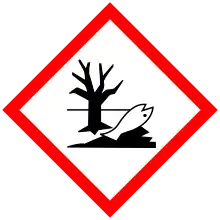 | |
| Warning | |
| H373, H410 | |
| P260, P273, P314, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Indaziflam is a preemergent herbicide especially for grass control in tree and bush crops.
History
In 1991, the Japanese company Idemitsu Kosan filed a patent to 2-amino 6-fluoroalkyl triazine derivatives as herbicides.[3] One of these compounds was subsequently given the ISO common name triaziflam but had limited success as a commercial herbicide.[4][5] Bayer scientists subsequently investigated this area of chemistry and identified indaziflam as having superior properties, which they patented and developed under the code number BCS-AA10717.[6][7] The compound was first registered for use in the USA in 2010.[8][9]
Mechanism of action
Indaziflam is an inhibitor of cellulose biosynthesis. This mechanism of action was theorized to be responsible for indaziflam's effect in 2009[7] and proven in 2014.[10] The cellulose biosynthesis inhibitors (CBIs) are identified as Class 29 by the Weed Science Society of America/Herbicide Resistance Action Committee.[11][12]
Resistance
As of March 2021 there are no resistant populations known[13] and none for the broader CBI class (discounting quinclorac).[11][14][15][16][17]
Brand names
Indaziflam composes all or part of the a.i. of several herbicides from Bayer Environmental Science (now owned by Cinven, aka Envu, per Bayer's and Envu's websites), [18] [19] including Rejuvra,[20] the Esplanade[21] line (sometimes mixed with diquat dibromide and glyphosate isopropylamine),[22] Marengo,[23][24] Specticle,[25][24] and Bayer CropScience (the inventor of the ingredient), like Alion.[26]
Uses
Indaziflam is approved in the United States for hops, Rubus spp., Coffea spp., bushberries, tropical crops, drupes/stone fruit, and tree nuts.[27] It is used as a preemergent.[28][27]
References
- ↑ Weed Science Society of America. "Common and chemical names approved by WSSA" (PDF).
- ↑ Pesticide Properties Database. "Indaziflam". University of Hertfordshire.
- ↑ US patent 5169425, Takematsu T.; Hirata T. & Kobayashi I. et al., "Herbicidal compositions comprising 2-Amino-4-Arylalkylamino-6-Haloalkyl-1,3,5-Triazines and Chlorophenoxy Acids and, optionally, substituted ureas", issued 1992-12-08, assigned to Idemitsu Kosan Company Limited
- ↑ Grossmann, Klaus; Tresch, Stefan; Plath, Peter (2001). "Triaziflam and Diaminotriazine Derivatives Affect Enantioselectively Multiple Herbicide Target Sites". Zeitschrift für Naturforschung C. 56 (7–8): 559–569. doi:10.1515/znc-2001-7-814. PMID 11531090. S2CID 13128483.
- ↑ "Triaziflam (ISO)". chem.nlm.nih.gov. Retrieved 21 March 2021.
- ↑ WO patent 2004069814, Ahrens H.; Dietrich H. & Minn K. et al., "Amino 1,3,5-Triazines N-substituted with chiral bicyclic radicals", issued 2004-08-19, assigned to Bayer Cropscience GMBH
- 1 2 Meyer, F.; Hanrahan, R.; Michel, J.; Monke, B.; Mudge, L.; Norton, L.; Olsen, C.; Parker, A.; Smith, J.; Spak, D. (2009). Indaziflam/BCS-AA10717-A new herbicide for pre-emergent control of grasses and broadleaf weeds for turf and ornamentals. WSSA Meeting Abstracts.
- ↑ Mark D. Parrish; R. Darren Unland; William J. Bertges (7–10 December 2009). "Introduction of Indaziflam for Weed Control in Fruit, Nut, and Grape Crops". North Central Weed Science Society Proceedings. Kansas City, Mo: North Central Weed Science Society. 64: 164.
- ↑ "Bayer CropSciences new herbicide indaziflam received first registration in U.S." Grainews. 6 September 2010. Retrieved 14 March 2021.
- ↑ Brabham, C.; Lei, L.; Gu, Y.; Stork, J.; Barrett, M.; DeBolt, S. (30 July 2014). "Indaziflam Herbicidal Action: A Potent Cellulose Biosynthesis Inhibitor". Plant Physiology. American Society of Plant Biologists (OUP). 166 (3): 1177–1185. doi:10.1104/pp.114.241950. ISSN 0032-0889. PMC 4226351. PMID 25077797. S2CID 12250466.
- 1 2 Weed Science Society of America (3 December 2020). "WSSA-Herbicide Site of Action (SOA) Classification List".
- ↑ Weed Science Society of America. "Summary of Herbicide Mechanism of Action According to the Weed Science Society of America (WSSA)" (PDF).
- ↑ "Herbicide Resistant Weeds by Individual Herbicide". International Survey of Herbicide Resistant Weeds. Herbicide Resistance Action Committee. Retrieved 14 March 2021.
- ↑ "List of Herbicide Resistant Weeds by Herbicide Mode of Action (L/26)". International Survey of Herbicide Resistant Weeds. Herbicide Resistance Action Committee. Retrieved 14 March 2021.
- ↑ Grossmann, Klaus; Kwiatkowski, Jacek (2000). "The Mechanism of Quinclorac Selectivity in Grasses". Pesticide Biochemistry and Physiology. Elsevier. 66 (2): 83–91. doi:10.1006/pest.1999.2461. ISSN 0048-3575. S2CID 84092985.
- ↑ Tresch, Stefan; Grossmann, Klaus (2003). "Quinclorac does not inhibit cellulose (cell wall) biosynthesis in sensitive barnyard grass and maize roots". Pesticide Biochemistry and Physiology. Elsevier. 75 (3): 73–78. doi:10.1016/s0048-3575(03)00013-0. ISSN 0048-3575. S2CID 84212641.
- ↑ Tresch, Stefan; Grossmann, Klaus (2003). "Erratum to "Quinclorac does not inhibit cellulose (cell wall) biosynthesis in sensitive barnyard grass and maize roots"". Pesticide Biochemistry and Physiology. Elsevier. 76 (2): 70–71. doi:10.1016/s0048-3575(03)00064-6. ISSN 0048-3575. S2CID 84794877.
- ↑ "Bayer completes sale of its Environmental Science Professional business to Cinven". Envu. Retrieved 11 October 2023.
- ↑ "Bayer completes sale of its Environmental Science Professional business to Cinven". Bayer Global. Retrieved 11 October 2023.
- ↑ "Envu's Rejuvra (indaziflam) Herbicide". Envu, formerly Bayer Environmental Science. Retrieved 8 October 2023.
- ↑ "Esplanade 200 SC IVM Product". Bayer Environmental Science US. Retrieved 14 March 2021.
- ↑ "Esplanade EZ IVM Product". Bayer Environmental Science US. Retrieved 14 March 2021.
- ↑ Spesard, Bruce (8 June 2018). "Broadleaf and Grassy Weed Control". Bayer Environmental Science. Retrieved 14 March 2021.
- 1 2 "Marengo (indaziflam) or Specticle". Extension Publications. NC State Ag Extension. 30 May 2014. Retrieved 14 March 2021.
- ↑ "Specticle Flo". Bayer Environmental Science. Retrieved 14 March 2021.
- ↑ "US84467332F (170705Fv2) ALION SC 32 FOZ ETL 0119.indd". CropScience. Bayer. 1 November 2019.
- 1 2 "PRIA Label Amendment – IR-4 tolerance petition and related amendments: (R190) to establish new uses on Hops, Caneberry subgroup 13-07A, Coffee, Bushberry subgroup 13-07B, Tropicals 23A and another amendment, and (R175) for crop group conversions in Stone Fruits 12-12 and Tree Nuts 14-12 Product Name: Indaziflam 200 SC Herbicide EPA Registration Number: 264-1106 Petition Number: 6E8452 Application Date: February 18, 2016 Decision Number(s): 514431, 514432, 514435, 514436" (PDF). US EPA. 5 July 2017.
- ↑ "Indaziflam". PubChem. NCBI, NLM, US NIH. Retrieved 14 March 2021. CID=CID 44146693 from PubChem