 | |
 | |
| Names | |
|---|---|
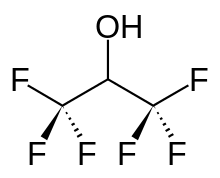
| Preferred IUPAC name
1,1,1,3,3,3-Hexafluoropropan-2-ol | |
| Other names
Hexafluoroisopropanol, Hexafluoroisopropyl alcohol, HFIP | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.011.873 |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H2F6O | |
| Molar mass | 168.038 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.596 g/mL |
| Melting point | −3.3 °C (26.1 °F; 269.8 K) |
| Boiling point | 58.2 °C (136.8 °F; 331.3 K) |
| Miscible | |
| Vapor pressure | 16 kPa at 20 °C |
| Viscosity | 1.65 cP at 20 °C |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H314, H361fd, H373 | |
| P201, P280, P303+P361+P353, P305+P351+P338+P310, P308+P313 | |
| NFPA 704 (fire diamond) | |
| Flash point | > 100 °C (212 °F; 373 K) |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
| Hexafluoroacetone; Isopropyl alcohol, 2,2,2-Trifluoroethanol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Hexafluoroisopropanol, commonly abbreviated HFIP, is the organic compound with the formula (CF3)2CHOH. This fluoroalcohol finds use as solvent in organic chemistry.[1] Hexafluoro-2-propanol is transparent to UV light with high density, low viscosity and low refractive index. It is a colorless, volatile liquid with a pungent odor.
Production
Hexafluoro-propan-2-ol is prepared from hexafluoropropylene through hexafluoroacetone, which is then hydrogenated.[2]
- (CF3)2CO + H2 → (CF3)2CHOH
Solvent properties
As a solvent, hexafluoro-2-propanol is polar and exhibits strong hydrogen bonding properties. Testament to the strength of its hydrogen-bonding tendency is the fact that its 1:1 complex with THF distills near 100 °C. It has a relatively high dielectric constant of 16.7. It is also relatively acidic, with a pKa of 9.3, comparable to that for phenol.[1] It is classified as a hard Lewis acid and its acceptor properties are discussed in the ECW model.[3][4]
Hexafluoro-propan-2-ol is a speciality solvent for organic synthesis, particularly for reactions involving oxidations and strong electrophiles. For example, HFIP enhances the reactivity of hydrogen peroxide as applied to Baeyer-Villiger oxidation of cyclic ketones.[1] In another illustration of its use, HFIP is used as the solvent for Lewis-acid catalyzed ring opening of epoxides.[5]
It has also found use in biochemistry to solubilize peptides and to monomerize β-sheet protein aggregates. Because of its acidity (pKa = 9.3), it can be used as acid in volatile buffers for ion pair HPLC – mass spectrometry of nucleic acids.[6] Recent studies[7] showed an ability of HFIP to activate allylic alcohols, stabilise an allylic cation, and further functionalize to allylic sulphides and sulfones.
Hexafluoro-propan-2-ol is a speciality solvent for some polar polymers.[8] It solubilizes polymers that are insoluble in common organic solvents, such as: polyamides, polyacrylonitriles, polyacetals, polyesters (e.g. polyglycolide), and polyketones. It has also been evaluated as a solvent for electrolysis.[9]
Medicine
It is both the precursor and the chief metabolite of the inhalation anesthetic sevoflurane. Sevoflurane gets metabolized within the body into HFIP and formaldehyde. HFIP is inactive, non-genotoxic and once formed, is rapidly conjugated with glucuronic acid and eliminated as a urinary metabolite.[10][11]
Safety
Toxicity
Hexafluoro-2-propanol has very low acute toxicity, hence its use as a precursor to anesthetics. Although it has low acute toxicity, it is a strong irritant to skin and eyes.[2] Animal experiments show possible adverse effects on fertility,[12] placing HFIP as a reproductive toxicity category 2 material.[13]
Environment and toxicity
HFIP is a specialty chemical that is produced in small quantities, thus it is not of significant environmental concern. Its environmental implications have been assessed.[14] HFIP also belongs to per- and polyfluorinated alkyl substances (PFAS).[15]
References
- 1 2 3 Colomer, Ignacio; Chamberlain, Anna E. R.; Haughey, Maxwell B.; Donohoe, Timothy J. (2017). "Hexafluoroisopropanol as a Highly Versatile Solvent". Nature Reviews Chemistry. 1 (11). doi:10.1038/s41570-017-0088.
- 1 2 Günter Siegemund, Werner Schwertfeger, Andrew Feiring, Bruce Smart, Fred Behr, Herward Vogel, Blaine McKusick “Fluorine Compounds, Organic” in Ullmann's Encyclopedia of Industrial Chemistry, John Wiley & Sons, 2007. doi:10.1002/14356007.a11_349
- ↑ Laurence, C.; Gal, J-F. (2010). Lewis Basicity and Affinity Scales, Data and Measurement. Wiley. p. 50-51. ISBN 978-0-470-74957-9.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Cramer, R. E.; Bopp, T. T. (1977). "Great E and C Plot. Graphical Display of the Enthalpies of Adduct Formation for Lewis Acids and Bases". Journal of Chemical Education. 54 (10): 612-613. doi:10.1021/ed054p612.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Travis W.Shaw, Julia A.Kalow, Abigail G.Doyle (2012). "Fluoride Ring-Opening Kinetic Resolution of Terminal Epoxides: Preparation of (S)-2-Fluoro-1-phenylethanol". Organic Syntheses. 89: 9. doi:10.15227/orgsyn.089.0009.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Apffel, A.; Chakel, J.A.; Fischer, S.; Lichtenwalter, K.; Hancock, W.S. (1997). "Analysis of oligonucleotides by HPLC-electrospray ionization mass spectrometry". Anal. Chem. 69 (7): 1320–1325. doi:10.1021/ac960916h. PMID 21639339.
- ↑ Lu, Maojian; Zhang, Rong-Jin; Zhu, Can-Ming; Xiao, Yonghong; Chen, Jian-Rui; Zhao, Lei-Min; Tong, Qing-Xiao; Zhong, Jian-Ji (October 2022). "HFIP-Induced Allylation Reaction of Tertiary Allylic Alcohols with Thiols or Sulfonyl Hydrazines to Access Allylic Organosulfurs". Synlett. 33 (17): 1745–1750. doi:10.1055/a-1915-8309. ISSN 0936-5214. S2CID 251303213.
- ↑ Lu, Le; Hua, Ruimao (20 May 2021). "A Monomer‐Polymer‐Monomer (MPM) Organic Synthesis Strategy: Synthesis and Application of Polybenzofuran for Functionalizing Benzene Ring of Benzofuran". Asian Journal of Organic Chemistry. 10 (8): 2137–2142. doi:10.1002/ajoc.202100208. S2CID 236388357.
- ↑ Ramos-Villaseñor, José Manuel; Rodríguez-Cárdenas, Esdrey; Barrera Díaz, Carlos E.; Frontana-Uribe, Bernardo A. (2020). "Review—Use of 1,1,1,3,3,3–hexafluoro–2–propanol (HFIP) Co-Solvent Mixtures in Organic Electrosynthesis". Journal of the Electrochemical Society. 167 (15): 155509. doi:10.1149/1945-7111/abb83c. S2CID 224972047.
- ↑ Baxter Healthcare Corporation (June 2017). "SEVOFLURANE- sevoflurane liquid DESCRIPTION". DailyMed. Retrieved 12 March 2021.
- ↑ "PubChem Compound Summary for CID 5206, Sevoflurane". PubChem. 2021. Retrieved 12 March 2021.
- ↑ "1,1,1,3,3,3-hexafluoropropan-2-ol Toxicity to Reproduction". ECHA. Retrieved 26 March 2021.
- ↑ "REGULATION (EC) No 1272/2008 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006". Official Journal of the European Union: 109. 31 December 2008. Retrieved 26 March 2021.
- ↑ Arp, Hans Peter H.; Hale, Sarah E. (November 2019). "REACH: Improvement of guidance and methods for the identification and assessment of PMT/vPvM substances". umweltbundesamt.de. Umweltbundesamt. Retrieved 12 March 2021.
- ↑ United States Environmental Protection Agency. "PFAS Master List of PFAS Substances (Version 2)". comptox.epa.gov/. Retrieved 12 March 2021.
Sources
- Radlick, Phillip C (1982-02-02). "Methods of synthesizing hexafluoroisopropanol from impure mixtures and synthesis of a fluoromethyl ether therefrom". United States Patent 4,314,087. Retrieved 2006-10-18.
- Cheminal, Bernard; H. Mathais; M. Thomarat (1987-03-03). "Process for the synthesis of 2,2,2-trifluoroethanol and 1,1,1,3,3,3-hexafluoroisopropanol". United States Patent 4,647,706. Retrieved 2006-10-18.
