 | |
| Names | |
|---|---|
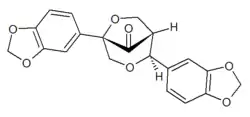
| Preferred IUPAC name
(1R,2S,5S)-2,5-Di(2H-1,3-benzodioxol-5-yl)-3,6-dioxabicyclo[3.2.1]octan-8-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C20H16O7 | |
| Molar mass | 368.341 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Gmelanone is a lignan found in the heartwood of Gmelina arborea.[1] Arboreol can be transformed by acid catalysis into gmelanone.[2]
References
- ↑ Anjaneyulu, A.S.R.; Rao, A.Madhusudhana; Rao, V.Kameswara; Row, L.Ramachandra; Pelter, Andrew; Ward, Robert S. (1977). "Novel hydroxy lignans from the heartwood of gmelina arborea". Tetrahedron. 33: 133–143. doi:10.1016/0040-4020(77)80444-4.
- ↑ Ramachandra Row, L.; Ventkateswarlu, Reveru; Pelter, Andrew; Ward, Robert S. (1980). "Acid catalysed rearrangements of arboreol: A biomimetic synthesis of gmelanone". Tetrahedron Letters. 21 (30): 2919. doi:10.1016/S0040-4039(00)78645-X.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.