 | |
| Clinical data | |
|---|---|
| Other names | BI 201335 |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
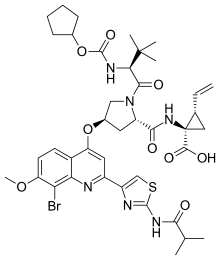
| Formula | C40H49BrN6O9S |
| Molar mass | 869.83 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Faldaprevir was an experimental drug for the treatment of hepatitis C (HCV). It was being developed by Boehringer-Ingelheim and reached Phase III clinical trials in 2011.[1] Boehringer announced in 2014 that it would not pursue approval of the drug any more because of better HCV treatments having become available.[2]
Mechanism of action
Faldaprevir is a hepatitis C virus protease inhibitor.
Studies
Faldaprevir was tested in combination regimens with pegylated interferon and ribavirin, and in interferon-free regimens with other direct-acting antiviral agents including deleobuvir.
Data from the SOUND-C2 study, presented at the 2012 AASLD Liver Meeting, showed that a triple combination of faldaprevir, deleobuvir, and ribavirin performed well in HCV genotype 1b patients.[3]
References
- ↑ Clinical trial number NCT01343888 for "Efficacy and Safety of BI 201335 (Faldaprevir) in Combination With Pegylated Interferon-alpha and Ribavirin in Treatment-naïve Genotype 1 Hepatitis C Infected Patients (STARTverso 1)" at ClinicalTrials.gov
- ↑ "Hepatitis C: Aus für Boehringers Faldaprevir". www.pharmazeutische-zeitung.de.
- ↑ Kanda T, Yokosuka O, Omata M (March 2015). "Faldaprevir for the treatment of hepatitis C". International Journal of Molecular Sciences. 16 (3): 4985–96. doi:10.3390/ijms16034985. PMC 4394460. PMID 25749475.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.