 | |
| Names | |
|---|---|
| IUPAC name
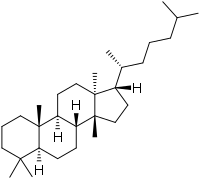
13α,14β-Lanostane | |
| Systematic IUPAC name
(1S,3aR,3bR,5aS,9aR,9bS,11aS)-3a,6,6,9a,11a-Pentamethyl-1-[(2R)-6-methylheptan-2-yl]hexadecahydro-1H-cyclopenta[a]phenanthrene | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| C30H54 | |
| Molar mass | 414.762 g·mol−1 |
| Density | 0.897 g/cm3[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Euphane is a tetracyclic triterpene that is the 13α,14β-stereoisomer of lanostane. Its derivatives are widely distributed in many plants.[2][3]
See also
References
- ↑ Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (© 1994-2019 ACD/Labs). Retrieved from SciFinder. [2019-10-19]
- ↑ Hou Y, Cao S, Brodie PJ, Miller JS, Birkinshaw C, Andrianjafy MN, Andriantsiferana R, Rasamison VE, TenDyke K, Shen Y, Suh EM, Kingston DG (2010). "Euphane triterpenoids of Cassipourea lanceolata from the Madagascar rainforest". Phytochemistry. 71 (5–6): 669–674. doi:10.1016/j.phytochem.2009.12.009. PMC 2847016. PMID 20074760.
- ↑ Wang LY, Wang NL, Yao XS, Miyata S, Kitanaka S (2003). "Euphane and tirucallane triterpenes from the roots of Euphorbia kansui and their in vitro effects on the cell division of Xenopus". J Nat Prod. 66 (5): 660–663. doi:10.1021/np0205396. PMID 12762796.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.