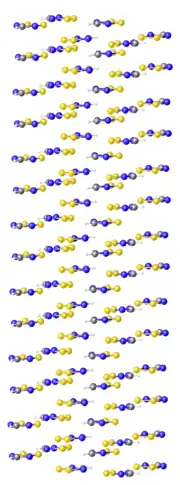
In chemistry, dithiadiazoles are a family of heterocyclic compounds with the formula RCN2S2. Two isomers have been studied: the 1,2‑dithia-3,5‑diazoles, in which the sulfur atoms are bonded to each other across the ring from the carbon atom, and the 1,3‑dithia-2,5‑diazoles, in which nitrogen and sulfur atoms alternate around the ring. In both cases, the neutral species are radicals that are of interest as examples of paramagnetic heterocycles. They have also attracted interest because of the tendency of the neutral species to form linear chain compounds, a theme in molecular electronics.
1,2-Dithia-3,5-diazoles and their oxidized derivatives
Structures
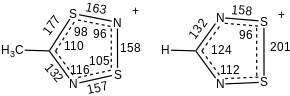
The structures of both the 1,2-dithia-3,5-diazolium cations and the neutral 1,2-dithia-3,5-diazoles are fairly similar. These are planar five-membered rings. With one electron in a π*-level, the neutral ring has longer bonds vs the cation. The elongation is a few percent. For example, the S–S distance in the neutral HCN2S2 is 207 vs 201 pm in the cation.[1] Melting cracks these dimers to produce a paramagnetic liquid.[4]: 177, 182 Dithiadiazole radicals equilibrate with their (diamagnetic) spin-paired dimers via weak S---S interactions. The neutral species' slight dipole moment places a positive charge on the sulfur atoms.[4]: 156
For the 1,2-dithia-3,5-diazolium salts, their bright colors vary with the R side chain and the counterion.[4]: 159–160
Synthesis
The traditional entry to dithiadiazoles is reduction the mono-cation RCN2S+
2. These cations are obtained by the addition of nitriles and thiazyl chloride:
- 2 RCN + 4 NSCl → 2 [RCN2S2]+Cl− + Cl2 + N2
For large-scale syntheses, a mixture of ammonium chloride and sulfur dichloride under a chlorine atmosphere substitutes cheaply for thiazyl chloride, but product yields are small. Alternatively, sulfur dichloride reacts with amidines to give dithiadiazolium chlorides, albeit with sensitivity to reaction conditions.[4]: 148–150
The dithidiazolium cations are reduced by treatment with iodide and thiocyanate:
- 2 [RCN2S2]+[I]- → 2 RCN2S•
2 + I2
A variety of reducing agents can be employed.[4]: 161, 174–175
Reactions


Halogens typically reoxidize dithiadiazoles to dithiazolium cations:
- 2 RCN2S•
2 + X2 → 2[ RCN2S2X +]X-
Dithiadiazoles react with low-valent metal ion by oxidative addition of the S–S bonds.[5] The products feature bidentate dithiolate-like ligands, each sulfur functioning as a bridging ligand.[4]: 192–194
Dithiadiazolium salts hydrolyze to amidine-hydrochloride salts, sulfur dioxide, and elemental sulfur.[4]: 171
1,3-Dithia-2,5-diazoles
1,3-Dithia-2,5-diazolium salts, which lack the S–S bond, are less common than the 1,2,3,5 isomer. The neutral 1,3-dithia-2,5-diazoles are even rarer.
The synthesis of these salts involves addition of dithionitronium hexafluoroarsenate ([SNS]+[AsF6]-) to a nitrile. The conversion is an example of a 1,3-Dipolar cycloaddition.[4]: 195–200
The sulfur atoms bear a slight positive charge.[4]: 201–202
The electrophilicity of dithiadiazolium salts is indicated by their tendency to polymerize THF. They hydrolyze to an amide through a poorly-understood mechanism.[4]: 206–209
The singly-reduced dithiazole radical is unstable, isomerizing to 1,2‑dithia-3,5‑diazole or forming uncharacterized brown polymers.[4]: 144, 204, 216–217
References
- 1 2 Cordes, A. W.; Bryan, C. D.; Davis, W. M.; De Laat, R. H.; Glarum, S. H.; Goddard, J. D.; Haddon, R. C.; Hicks, R. G.; Kennepohl, D. K. (1993). "Prototypal 1,2,3,5-dithia- and -diselenadiazolyl [HCN2E2]• (E = sulfur, selenium): Molecular and electronic structures of the radicals and their dimers, by theory and experiment". Journal of the American Chemical Society. 115 (16): 7232–7239. doi:10.1021/ja00069a022.
- ↑ MacLean, Gregory K.; Passmore, Jack; Rao, M. N. Sudheedra; Schriver, Melbourne J.; White, Peter S.; Bethell, Donald; Pilkington, Roger S.; Sutcliffe, Leslie H. (1985). "Preparation of 1,3,2-dithiazolium hexafluoroarsenate(V), preparation and crystal structures of 5-methyl-1,3,2,4-dithiadiazolium and 4-methyl-1,3,2-dithiazolium hexafluoroarsenate(V) and the reduction of these salts to stable free radicals". Journal of the Chemical Society, Dalton Transactions (7): 1405. doi:10.1039/dt9850001405.
- ↑ Bryan, C. D.; Cordes, A. W.; Fleming, R. M.; George, N. A.; Glarum, S. H.; Haddon, R. C.; MacKinnon, C. D.; Oakley, R. T.; Palstra, T. T. M.; Perel, A. S. (1995). "Charge Transfer Salts of Benzene-Bridged 1,2,3,5-Dithiadiazolyl Diradicals. Preparation, Structures, and Transport Properties of 1,3- and 1,4-[(S2N2C)C6H4(CN2S2)][X] (X = I, Br)". Journal of the American Chemical Society. 117 (26): 6880–6888. doi:10.1021/ja00131a009. S2CID 96841129.
- 1 2 3 4 5 6 7 8 9 10 11 Rawson, Jeremy M.; Banister, Arthur J.; Lavender, Ian (1995). "The Chemistry of Dithiadiazolylium and Dithiadiazolyl Rings". Adv. Heterocyc. Chem. 62. doi:10.1016/S0065-2725(08)60422-5.
- ↑ Lau, Hiu Fung; Ang, Pearly Chwee Ying; Ng, Victor Wee Lin; Kuan, Seah Ling; Goh, Lai Yoong; Borisov, Alexey S.; Hazendonk, Paul; Roemmele, Tracey L.; Boeré, René T.; Webster, Richard D. (2008). "Coupling of CpCr(CO)3 and Heterocyclic Dithiadiazolyl Radicals. Synthetic, X-ray Diffraction, Dynamic NMR, EPR, CV, and DFT Studies". Inorganic Chemistry. 47 (2): 632–644. doi:10.1021/ic702128f. PMID 18161967.