 A cartoon of Methanococcus jannaschii diaminopimelate decarboxylase | |||||||||
| Identifiers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| EC no. | 4.1.1.20 | ||||||||
| CAS no. | 9024-75-3 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
The enzyme diaminopimelate decarboxylase (EC 4.1.1.20) catalyzes the cleavage of carbon-carbon bonds in meso 2,6 diaminoheptanedioate to produce CO2 and L-lysine, the essential amino acid. It employs the cofactor pyridoxal phosphate, also known as PLP, which participates in numerous enzymatic transamination, decarboxylation and deamination reactions.[1]
This enzyme belongs to the family of lyases, specifically the carboxy-lyases, which cleave carbon-carbon bonds. The systematic name of this enzyme class is meso-2,6-diaminoheptanedioate carboxy-lyase (L-lysine-forming).DAP-decarboxylase catalyzes the final step in the meso-diaminopimelate/lysine biosynthetic pathway.[2] Lysine is used for protein synthesis and used in the peptidoglycan layer of Gram-positive bacteria cell walls.[2] This enzyme is not found in humans, but the ortholog is ornithine decarboxylase.[3]
Structure
DAPDC is a PLP-dependent enzyme belonging to the alanine racemase family.[4] This enzyme is generally dimeric with each monomer containing two domains.[5] The first domain is the N-terminal α/β-barrel that binds the PLP to the active site lysine residue.[3][4][5] The second domain is the C-terminal β-sandwich.[4][5] The active site is formed from residues present in both domains resulting in two active sites within the dimer.[5]
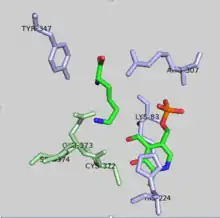
DAPDC is stereochemically specific due to the opposing chiralities at each terminus of diaminopimelate.[5] In order for the L-lysine to be generated over D-lysine, decarboxylation must occur at the D-terminus. Whether DAPDC recognizes the terminus or not is dependent on the formation of a Schiff base with PLP.[5]
While the majority of DAPDC found in various species of bacteria have the same basic components, not all species follow the same structure.[3] Some species of bacteria, such as Mycobacterium tuberculosis have been observed as a tetramer.[6] The tetramer is shaped like a ring with the active sites accessible from the inside of the enzyme.[6]
Mechanism
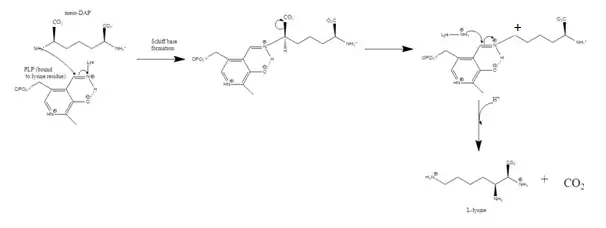
The first step in the mechanism is the same as for all type III PLP-dependent enzymes; the formation of a Schiff base with the substrate amino group.[5] The lysine residue binding PLP to the structure is replaced by diaminopimelate.[4][7] DAPDC then uses the interaction of 3 residues (Arginine, Aspartate, and Glutamate) within the active site to identify the D-stereocenter.[3][7] The DAP is decarboxylated and then stabilized by PLP.[4] It is not clear which general acid protonates after decarboxylation, but there is speculation that the lysine residue is the donor.[7]
Regulation
DAPDC is regulated by the product L-lysine at relatively high concentrations.[3][8] Compounds that are similar to DAP in chemical complexity do not inhibit the reaction, possibly due to the residue rulers creating specific bond angles.[3] Diamines have a stronger inhibitory effect compared to dicarboxylic acids, most likely from interactions with PLP.[3]
Function
Given that there are three pathways to convert aspartate to lysine, this is clearly an essential process for the cell, particularly in building cell walls in Gram-positive bacteria.[2][9] There is no process for producing lysine in humans, but ornithine decarboxylase shares many similarities with DAPDC.[4] Both enzymes use PLP as a cofactor and have similar structures forming the active sites.[7] However, DAPDC differs in that it decarboxylates at the D-stereocenter and is highly stereospecific.[7] These unique features make DAPDC a good candidate for antibacterial studies because potential inhibitors of such an integral step in cell viability would be unlikely to interact with necessary processes within humans.
References
- ↑ "Pyridoxal phosphate". Pubchem. Retrieved 2018-03-09.
- 1 2 3 Gillner DM, Becker DP, Holz RC (February 2013). "Lysine biosynthesis in bacteria: a metallodesuccinylase as a potential antimicrobial target". Journal of Biological Inorganic Chemistry. 18 (2): 155–63. doi:10.1007/s00775-012-0965-1. PMC 3862034. PMID 23223968.
- 1 2 3 4 5 6 7 Peverelli MG, Soares da Costa TP, Kirby N, Perugini MA (April 2016). "Dimerization of Bacterial Diaminopimelate Decarboxylase Is Essential for Catalysis". The Journal of Biological Chemistry. 291 (18): 9785–95. doi:10.1074/jbc.M115.696591. PMC 4850314. PMID 26921318.
- 1 2 3 4 5 6 Kidron H, Repo S, Johnson MS, Salminen TA (January 2007). "Functional classification of amino acid decarboxylases from the alanine racemase structural family by phylogenetic studies". Molecular Biology and Evolution. 24 (1): 79–89. doi:10.1093/molbev/msl133. PMID 16997906.
- 1 2 3 4 5 6 7 Ray SS, Bonanno JB, Rajashankar KR, Pinho MG, He G, De Lencastre H, Tomasz A, Burley SK (November 2002). "Cocrystal structures of diaminopimelate decarboxylase: mechanism, evolution, and inhibition of an antibiotic resistance accessory factor". Structure. 10 (11): 1499–508. doi:10.1016/S0969-2126(02)00880-8. PMID 12429091.
- 1 2 Weyand S, Kefala G, Svergun DI, Weiss MS (September 2009). "The three-dimensional structure of diaminopimelate decarboxylase from Mycobacterium tuberculosis reveals a tetrameric enzyme organisation". Journal of Structural and Functional Genomics. 10 (3): 209–17. doi:10.1007/s10969-009-9065-z. PMID 19543810. S2CID 212206.
- 1 2 3 4 5 Fogle EJ, Toney MD (September 2011). "Analysis of catalytic determinants of diaminopimelate and ornithine decarboxylases using alternate substrates". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 1814 (9): 1113–9. doi:10.1016/j.bbapap.2011.05.014. PMC 3124589. PMID 21640851.
- ↑ Rosner A (January 1975). "Control of lysine biosynthesis in Bacillus subtilis: inhibition of diaminopimelate decarboxylase by lysine". Journal of Bacteriology. 121 (1): 20–8. doi:10.1128/JB.121.1.20-28.1975. PMC 285608. PMID 234936.
- ↑ Dogovski C, Atkinson SC, Dommaraju SR, Dobson RC, Perugini MA (2009). "Lysine biosynthesis in bacteria – an unchartered pathway for novel antibiotic design" (PDF). Biotechnology. XI: 146–166.
Further reading
- Denman RF, Hoare DS, Work E (March 1955). "Diaminopimelic acid decarboxylase in pyridoxin-deficient Escherichia coli". Biochimica et Biophysica Acta. 16 (3): 442–3. doi:10.1016/0006-3002(55)90257-2. PMID 14378182.