In chemistry, a dehydration reaction is a chemical reaction that involves the loss of water from the reacting molecule or ion. Dehydration reactions are common processes, the reverse of a hydration reaction.
Dehydration reactions in organic chemistry
Esterification
The classic example of a dehydration reaction is the Fischer esterification, which involves treating a carboxylic acid with an alcohol to give an ester
- RCO2H + R′OH ⇌ RCO2R′ + H2O
Often such reactions require the presence of a dehydrating agent, i.e. a substance that reacts with water.
Etherification
Two monosaccharides, such as glucose and fructose, can be joined together (to form saccharose) using dehydration synthesis. The new molecule, consisting of two monosaccharides, is called a disaccharide.
Nitrile formation
Nitriles are often prepared by dehydration of primary amides.
- RC(O)NH2 → RCN + H2O
Ketene formation
Ketene is produced by heating acetic acid and trapping the product:[1]
- CH3CO2H → CH2=C=O + H2O
Alkene formation
Alkenes can be made from alcohols by dehydration. This conversion, among others, is used in converting biomass to liquid fuels.[2] The conversion of ethanol to ethylene is a fundamental example:[3][4]
- CH3CH2OH → H2C=CH2 + H2O
The reaction is accelerated by acid catalysts such as sulfuric acid and certain zeolites. These reactions often proceed via carbocation]]ic intermediates as shown for the dehydration of cyclohexanol.[5]
Some alcohols are prone to dehydration. 3-Hydroxylcarbonyls, called aldols, release water upon standing at room temperature:
- RC(O)CH2CH(OH)R' → RC(O)CH=CHR' + H2O
The reaction is induced by dehydrating reagents. For example, 2-methyl-cyclohexan-1-ol dehydrates to 1-methylcyclohexene in the presence of Martin's sulfurane, which reacts irreversibly with water.[6][7]
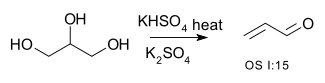
Double dehydration is illustrated by the conversion of glycerol to acrolein:[8][9]
Dehydration reactions in inorganic chemistry
The formation of the pyrophosphate bond is an important dehydration relevant to bioenergetics.
Various construction materials are produced by dehydration. Plaster of Paris is produced by dehydration of gypsum in a kiln:[10][11]
- heat (released as steam).
The resulting dry powder is ready to be mixed with water to form a stiff but workable paste that hardens.
See also
References
- ↑ Miller, Raimund; Abaecherli, Claudio; Said, Adel; Jackson, Barry (2001). "Ketenes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_063. ISBN 978-3527306732.
- ↑ Besson, Michèle; Gallezot, Pierre; Pinel, Catherine (2014-02-12). "Conversion of Biomass into Chemicals over Metal Catalysts". Chemical Reviews. 114 (3): 1827–1870. doi:10.1021/cr4002269. ISSN 0009-2665. PMID 24083630.
- ↑ Zimmermann, Heinz; Walz, Roland (2008). "Ethylene". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_045.pub3. ISBN 978-3527306732.
- ↑ Zhang, Minhua; Yu, Yingzhe (2013-07-17). "Dehydration of Ethanol to Ethylene". Industrial & Engineering Chemistry Research. 52 (28): 9505–9514. doi:10.1021/ie401157c. ISSN 0888-5885.
- ↑ G. H. Coleman, H. F. Johnstone (1925). "Cyclohexene". Organic Syntheses. 5: 33. doi:10.15227/orgsyn.005.0033.
- ↑ J. Brent Friesen; Robert Schretzman (2011). "Dehydration of 2-Methyl-1-cyclohexanol: New Findings from a Popular Undergraduate Laboratory Experiment". J. Chem. Educ. 88 (8): 1141–1147. Bibcode:2011JChEd..88.1141F. doi:10.1021/ed900049b.
- ↑ Roden, Brian A. (2001). "Diphenylbis(1,1,1,3,3,3-hexafluoro-2-phenyl-2-propoxy)sulfurane". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd409. ISBN 0471936235.
- ↑ H. Adkins; W. H. Hartung (1926). "Acrolein". Organic Syntheses. 6: 1. doi:10.15227/orgsyn.006.0001.
- ↑ Katryniok, Benjamin; Paul, Sébastien; Bellière-Baca, Virginie; Rey, Patrick; Dumeignil, Franck (2010). "Glycerol dehydration to acrolein in the context of new uses of glycerol". Green Chemistry. 12 (12): 2079. doi:10.1039/c0gc00307g. ISSN 1463-9262.
- ↑ Franz Wirsching "Calcium Sulfate" in Ullmann's Encyclopedia of Industrial Chemistry, 2012 Wiley-VCH, Weinheim. doi:10.1002/14356007.a04_555
- ↑ Staff. "CaSO4, ½ H2O". LaFargePrestia. Archived from the original on November 20, 2008. Retrieved 27 November 2008.