 | |
| Names | |
|---|---|
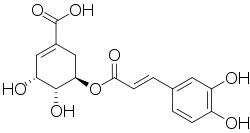
| Preferred IUPAC name
(3R,4R,5R)-3-{[(2E)-3-(3,4-Dihydroxyphenyl)prop-2-enoyl]oxy}-4,5-dihydroxycyclohex-1-ene-1-carboxylic acid | |
| Other names
Dattelic acid; 5-O-Caffeoylshikimic acid; trans-5-O-Caffeoylshikimic acid; 5-Caffeoylshikimic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C16H16O8 | |
| Molar mass | 336.296 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Dactylifric acid (also known as dattelic acid or 5-O-caffeoylshikimic acid[2][3][4]) is an ester derived from caffeic acid and shikimic acid. It and its isomers are enzymic browning substrates found in dates (Phoenix dactylifera fruits).[3][5]
Some older sources identify dactylifric acid as 3-O-caffeoylshikimic acid.[5]

Chemical structure of 3-O-caffeoylshikimic acid
References
- ↑ "5-O-Caffeoylshikimic acid". CAS Common Chemistry.
- ↑ Fukuoka, Masamichi (1982). "Chemical and toxicological studies on bracken fern, Pteridium aquilinum var. Latiusculum. VI. Isolation of 5-O-caffeoylshikimic acid as an antithiamine factor". Chemical and Pharmaceutical Bulletin. 30 (9): 3219–3224. doi:10.1248/cpb.30.3219. PMID 6926750.
5-O-Caffeoylshikimic acid (dactylifric acid) was isolated...
- 1 2 Ziouti, A.; Modafar, C.; Fleuriet, A.; Boustani, S.; Macheix, J. J. (1996). "Phenolic compounds in date palm cultivars sensitive and resistant to Fusarium oxysporum". Biologia Plantarum. 38 (3): 451–457. doi:10.1007/BF02896679. S2CID 38035795.
5-caffeoylshikimic acid (dactylifric acid) and its positional isomers (3-caffeoylshikimic acid and 4-caffeoylshikimic acid)...
- ↑ "Chlorogenic acids and the acyl-quinic acids: discovery, biosynthesis, bioavailability and bioactivity" (PDF).
Trivial Name: Dactylifric acid ... Current Interpretation with IUPAC numbering: 5-O-Caffeoylshikimic acid
- 1 2 Maier, V. P.; Metzler, D. M.; Huber, A. F. (1964). "3-O-Caffeoylshikimic acid (dactylifric acid) and its isomers, a new class of enzymic browning substrates". Biochemical and Biophysical Research Communications. 14 (2): 124–128. doi:10.1016/0006-291x(64)90241-4. PMID 5836492.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.