 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Cyclohexylsulfamic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.635 |
| E number | E952(i) (glazing agents, ...) |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
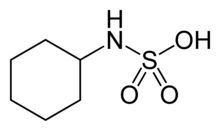
| C6H13NO3S | |
| Molar mass | 179.23 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Cyclamic acid is a compound with formula C6H13NO3S.
It is included in E number "E952".
Cyclamic acid is mainly used as catalyst in the production of paints and plastics, and furthermore as a reagent for laboratory usage.[1]
The sodium and calcium salts of cyclamic acid are used as artificial sweeteners under the name cyclamate.[2]
References
- ↑ Johnson, Darryl E; Nunn, Helmut B; Bruckenstein, Stanley (2002). "Quantitative hydrolysis of sodium cyclamate and calcium cyclamate to cyclohexylamine, followed by colorimetric analysis". Analytical Chemistry. 40 (2): 368–370. doi:10.1021/ac60258a033.
- ↑ Chattopadhyay, Sanchari; Raychaudhuri, Utpal; Chakraborty, Runu (2011). "Artificial sweeteners – a review". Journal of Food Science and Technology. 51 (4): 611–621. doi:10.1007/s13197-011-0571-1. PMC 3982014. PMID 24741154.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.