In biochemistry, chelatases are enzymes that catalyze the insertion ("metalation") of naturally occurring tetrapyrroles. Many tetrapyrrole-based cofactors exist in nature including hemes, chlorophylls, and vitamin B12. These metallo cofactors are derived by the reaction of metal cations with tetrapyrroles, which are not ligands per se, but the conjugate acids thereof. In the case of ferrochelatases, the reaction that chelatases catalyze is:[1]
- Fe2+ + H2P → FeP + 2 H+
In the above equation H2P represents a sirohydrochlorin or a porphyrin, such as protoporphyrin IX.
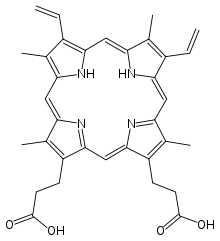
Chelatases are required because porphyrins and related macrocyclic ligands are extremely slow to metalate, despite favorable thermodynamics. These low rates are attributed to the tight fit of the metal into the rigid 18- or 17-membered tetrapyrrole macrocycle. Several families of chelatase are known including cobalt chelatase, magnesium chelatase, and ferrochelatase. Nickel insertion into a sirohydrochlorin also requires a chelatase as part of the biosynthesis of cofactor F430. Apparently that chelatase is identical to the cobalt chelatase.[2]
References
- ↑ Kaushik Saha; Michaël Moulin; Alison G. Smith (2009). "Tetrapyrroles in Plants: Chemical Biology of Metal Insertion and Removal". Wiley Encyclopedia of Chemical Biology. Encyclopedia of Chemical Biology. John Wiley & Sons. doi:10.1002/9780470048672.wecb454. ISBN 978-0470048672.
- ↑ Moore, Simon J.; Sowa, Sven T.; Schuchardt, Christopher; Deery, Evelyne; Lawrence, Andrew D.; Ramos, José Vazquez; Billig, Susan; Birkemeyer, Claudia; Chivers, Peter T. (2017-03-02). "Elucidation of the biosynthesis of the methane catalyst coenzyme F430". Nature. 543 (7643): 78–82. doi:10.1038/nature21427. ISSN 0028-0836. PMC 5337119. PMID 28225763.